Análisis de las afinidades de unión de la ivermectina, las proteínas nucleocápside y ORF6 del SARS-CoV-2 a las isoformas de las importinas α humanas: Un enfoque computacional
DOI:
https://doi.org/10.18633/biotecnia.v27.2485Palabras clave:
antiparasitario, quimioinformática, carioferinas, proteínas virales, COVID-19Resumen
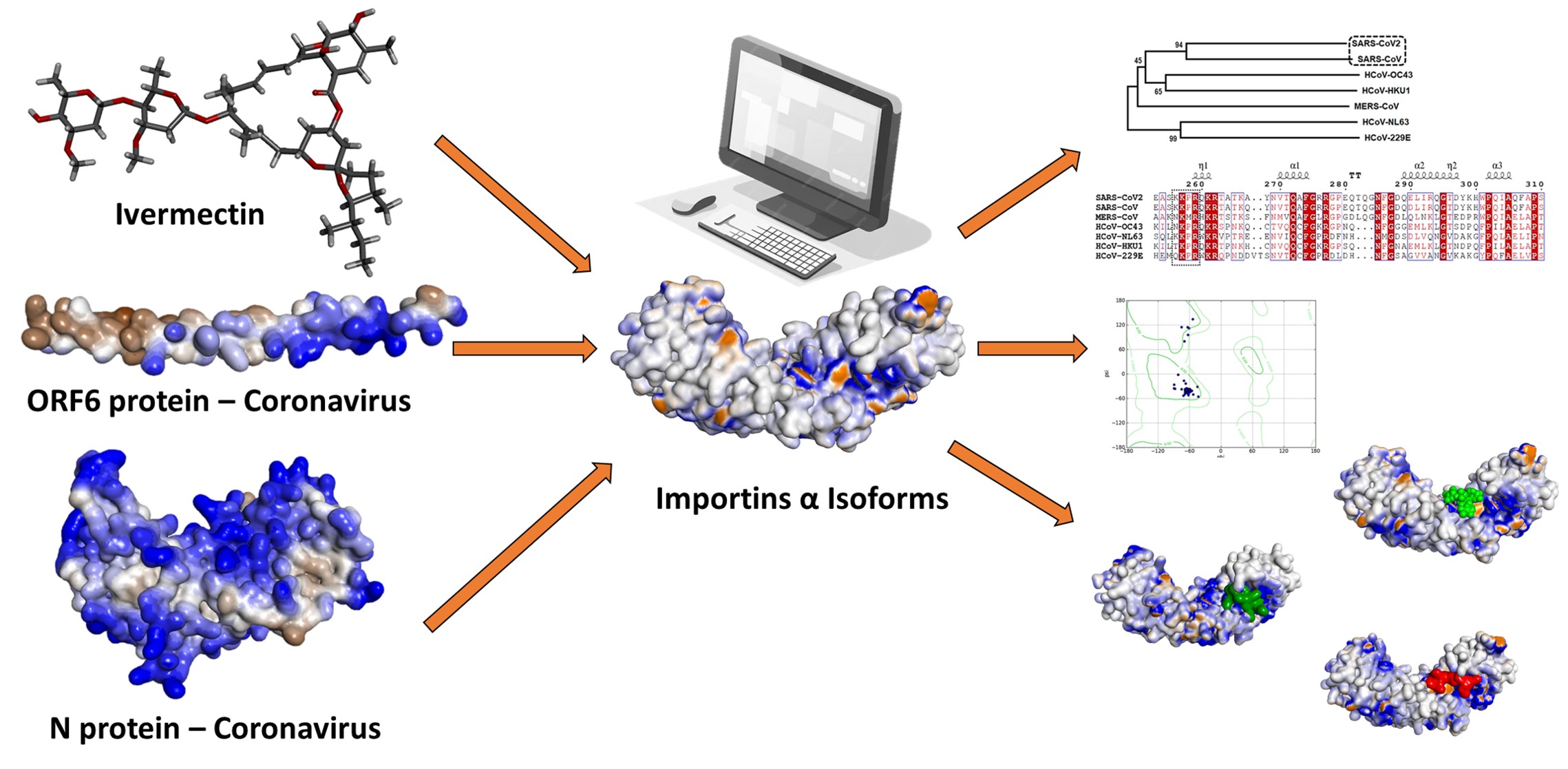
Se ha demostrado in vitro que la ivermectina reduce la replicación del SARS-CoV-2 en células infectadas mediante la interacción con importinas α, sin embargo, aún se desconoce el mecanismo exacto de acción. El objetivo de este estudio fue analizar afinidades de unión de la ivermectina, la nucleocápside (N) y las proteínas ORF6 del SARS-CoV-2 con isoformas de importinas α humanas utilizando métodos de acoplamiento molecular. Se utilizaron estructuras cristalizadas de importinas α de Protein Data Bank (PDB) y de la AlphaFold Protein Structure Database, y las proteínas virales se modelaron utilizando AlphaFold 2. Se llevaron a cabo simulaciones de acoplamiento molecular entre isoformas de la importina α humana, la ivermectina, y las proteínas N y ORF6, empleando los algoritmos Broy-den-Fletcher-Goldfarb-Shanno, FTDock y pyDockRST. Los datos obtenidos evidenciaron que las proteínas virales del SARS-CoV-2 y la ivermectina presentan afinidades de unión favorables a los dominios ARM2-ARM4 (sitio principal de unión), compartiendo afinidades de interacción con los mismos residuos activos. Estos resultados sugieren que la ivermectina comparte el mismo sitio activo en las importinas α que las proteínas N y ORF6 de SARS-CoV-2, demostrando una potencial diana molecular para la investigación en el desarrollo de nuevos fármacos antivirales contra la COVID-19.
Descargas
Citas
Addetia, A., Lieberman, N. A. P., Phung, Q., Hsiang, T.Y., Xie, H., Roychoudhury, P., Shrestha, L., Loprieno, M. A, Huang, M. L., Gale, M. J., Jerome, K. R. and Greninger, A. L. 2021. SARS-CoV-2 ORF6 Disrupts Bidirectional Nucleocytoplasmic Transport through Interactions with Rae1 and Nup98. mBio, 12(2), e00065-21. https://doi.org/10.1128/mBio.00065-21
Andersen, K. G., Rambaut, A., Lipkin, W. I., Holmes, E. C. and Garry, R. F. 2020. The proximal origin of SARS-CoV-2. Nature Medicine, 26(4), 450-452. https://doi.org/10.1038/s41591-020-0820-9
Azam, F., Taban, I. M., Eid, E. E. M., Iqbal, M., Alam, O., Khan, S., Mahmood, D., Anwar, M. J., Khaililullah, H. and Khan, M. U. 2020. An in-silico analysis of ivermectin interaction with potential SARS-CoV-2 targets and host nuclear importin α. Journal of Biomolecular Structure and Dynamics, 40(6), 2851–2864. https://doi.org/10.1080/07391102.2020.1841028
Baumhardt, J. and Chook, Y. M. 2018. Structures of Importins and Exportins. En W. Yang (Ed.), Nuclear-Cytoplasmic Transport (pp. 113-149). Cham: Springer International Publishing. https://doi.org/10.1007/978-3-319-77309-4_6
Bello, M. 2022. Elucidation of the inhibitory activity of ivermectin with host nuclear importin α and several SARS-CoV-2 targets. Journal of Biomolecular Structure and Dynamics, 40(18), 8375-8383. https://doi.org/10.1080/07391102.2021.1911857
Berman, H. M., Westbrook, J., Feng, Z., Gilliland, G., Bhat, T. N., Weissig, H., Shindyalov, I. N. and Bourne, P. E. 2000. The Protein Data Bank. Nucleic Acids Research, 28(1), 235-242. https://doi.org/10.1093/nar/28.1.235
Bian, X.L. and Wilson, V. G. 2010. Common importin alpha specificity for papillomavirus E2 proteins. Virus Research, 150(1), 135-137. https://doi.org/10.1016/j.virusres.2010.02.011
Caly, L., Druce, J. D., Catton, M. G., Jans, D. A. and Wagstaff, K. M. 2020. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Research, 178, 104787. https://doi.org/10.1016/j.antiviral.2020.104787
Ceraolo, C. and Giorgi, F. M. 2020. Genomic variance of the 2019-nCoV coronavirus. Journal of Medical Virology, 92(5), 522-528. https://doi.org/10.1002/jmv.25700
Chang, C., Hou, M.H., Chang, C.F., Hsiao, C.D. and Huang, T. 2014. The SARS coronavirus nucleocapsid protein – Forms and functions. Antiviral Research, 103, 39-50. https://doi.org/10.1016/j.antiviral.2013.12.009
Chelliah, V., Blundell, T. L. and Fernández-Recio, J. 2006. Efficient Restraints for Protein–Protein Docking by Comparison of Observed Amino Acid Substitution Patterns with those Predicted from Local Environment. Journal of Molecular Biology, 357(5), 1669-1682. https://doi.org/10.1016/j.jmb.2006.01.001
Cheng, T. M.K., Blundell, T. L. and Fernandez-Recio, J. 2007. pyDock: Electrostatics and desolvation for effective scoring of rigid-body protein–protein docking. Proteins: Structure, Function, and Bioinformatics, 68(2), 503-515. https://doi.org/10.1002/prot.21419
Chook, Y. and Blobel, G. 2001. Karyopherins and nuclear import. Current Opinion in Structural Biology, 11(6), 703-715. https://doi.org/10.1016/S0959-440X(01)00264-0
Choudhury, A., Das, N. C., Patra, R., Bhattacharya, M., Ghosh, P., Patra, B. C. and Mukherjee, S. 2021. Exploring the binding efficacy of ivermectin against the key proteins of SARS-CoV-2 pathogenesis: An in silico approach. Future Virology, 16(4), 277-291. https://doi.org/10.2217/fvl-2020-0342
Crump, A. 2017. Ivermectin: Enigmatic multifaceted ‘wonder’ drug continues to surprise and exceed expectations. The Journal of Antibiotics, 70(5), 495-505. https://doi.org/10.1038/ja.2017.11
Dallakyan, S. and Olson, A. J. 2015. Small-Molecule Library Screening by Docking with PyRx. En J. E. Hempel, C. H. Williams, and C. C. Hong (Eds.), Chemical Biology: Methods and Protocols (pp. 243-250). New York, NY: Springer. https://doi.org/10.1007/978-1-4939-2269-7_19
David, A., Islam, S., Tankhilevich, E. and Sternberg, M. J. E. 2022. The AlphaFold Database of Protein Structures: A Biologist’s Guide. Journal of Molecular Biology, 434(2), 167336. https://doi.org/10.1016/j.jmb.2021.167336
Fagerlund, R., Melén, K., Kinnunen, L. and Julkunen, I. 2002. Arginine/Lysine-rich Nuclear Localization Signals Mediate Interactions between Dimeric STATs and Importin α5 *. Journal of Biological Chemistry, 277(33), 30072-30078. https://doi.org/10.1074/jbc.M202943200
Fontes, M. R. M., Teh, T. and Kobe, B. 2000. Structural basis of recognition of monopartite and bipartite nuclear localization sequences by mammalian importin-α11Edited by K. Nagai. Journal of Molecular Biology, 297(5), 1183-1194. https://doi.org/10.1006/jmbi.2000.3642
Frieman, M., Yount, B., Heise, M., Kopecky-Bromberg, S. A., Palese, P. and Baric, R. S. 2007. Severe Acute Respiratory Syndrome Coronavirus ORF6 Antagonizes STAT1 Function by Sequestering Nuclear Import Factors on the Rough Endoplasmic Reticulum/Golgi Membrane. Journal of Virology, 81(18), 9812-9824. https://doi.org/10.1128/JVI.01012-07
Gabb, H. A., Jackson, R. M. and Sternberg, M. J. E. 1997. Modelling protein docking using shape complementarity, electrostatics and biochemical information11Edited by J. Thornton. Journal of Molecular Biology, 272(1), 106-120. https://doi.org/10.1006/jmbi.1997.1203
Gao, T., Gao, Y., Liu, X., Nie, Z., Sun, H., Lin, K., Peng, H. and Wang, S. 2021. Identification and functional analysis of the SARS-COV-2 nucleocapsid protein. BMC Microbiology, 21(1), 58. https://doi.org/10.1186/s12866-021-02107-3
Gayozo, E., Rojas, L. and López, M. 2020. Análisis computacional de la interacción in silico de la Agatisflavona con la región de trimerización de la proteína espícula (S) del SARS-CoV-2. Steviana, 12(2), 70-80. https://doi.org/10.56152/StevianaFacenV12N2A4_2020
Gayozo, E. and Rojas, L. 2021. Interacción in silico de las moléculas Agathisflavona, Amentoflavona y Punicalina con la Importina α1 humana. Revista Colombiana de Biotecnología, 23(2), 15-24. https://doi.org/10.15446/rev.colomb.biote.v23n2.94466
Gordon, D. E., Jang, G. M., Bouhaddou, M., Xu, J., Obernier, K., White, K. M., O’Meara, M. J., Rezelj, V. V., Guo, J. Z., Swaney, D. L., Tummino, T. A., Hüttenhain, R., Kaake, R. M., Richards, A. L., Tutuncuoglu, B., Foussard, H., Batra, J., Haas, K., Modak, M., Kim, M., Haas, P., Polacco, B. J., Braberg, H., Fabius, J. M., Eckhardt, M., Soucheray, M., Bennett, M. J., Cakir, M., McGregor, M. J., Li, Q., Meyer, B., Roesch, F., Vallet, T., Mac Kain, A., Miorin, L., Moreno, E., Chi Naing, Z. Z., Zhou, Y., Peng, S., Shi, Y., Zhang, Z., Shen, W., Kirby, I. T., Melnyk, J. E., Chorba, J. S., Lou, K., Dai, S. A., Barrio-Hernandez, I., Memon, D., Hernandez-Armenta, C., Lyu, J., Mathy, C. P., Perica, T., Bharath Pilla, K., Ganesan, S. J., Saltzberg, D. J., Rakesh, R., Liu, X., Rosenthal, S. B., Calviello, L., Venkataramanan, S., Liboy-Lugo, J., Lin, Y., Huang, X. P., Liu, Y., Wankowicz, S. A., Bohn, M., Safari, M., Ugur, F. S., Koh, C., Savar, N. S., Dinh Tran, Q., Shengjuler,D., Fletcher, S. J., O’Neal, M. C., Cai, Y., Chang, J. C., Broadhurst, D. J., Klippsten, S., Sharp, P. P., Wenzell, N. A., Kuzuoglu-Ozturk, D., Wang, H., Trenker, R., Young, J. M., Cavero, D. A., Hiatt, J., Roth, T. L., Rathore, U., Subramanian, A., Noack, J., Hubert, M., Stroud, R. M., Frankel, A. D., Rosenberg, O. S., Verba, K. A., Agard, D. A., Ott, M., Emerman, M., Jura, N., von Zastrow, M., Verdin, E., Ashworth, A., Schwartz, O., d’Enfert, C., Mukherjee, S., Jacobson, M., Malik, H. M., Fujimori, D. G., Ideker, T., Craik, C. S., Floor, S. N., Fraser, J. S., Gross, J. D., Sali, A., Roth, B. L., Ruggero, D., Taunton, J., Kortemme, T., Beltrao, P., Vignuzzi, M., García-Sastre, A., Shokat, K. M., Shoichet, B. K. and Krogan, N. J. 2020. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature, 583(7816), 459-468. https://doi.org/10.1038/s41586-020-2286-9
Grosdidier, S., Pons, C., Solernou, A. and Fernández-Recio, J. 2007. Prediction and scoring of docking poses with pyDock. Proteins: Structure, Function, and Bioinformatics, 69(4), 852-858. https://doi.org/10.1002/prot.21796
He, R., Dobie, F., Ballantine, M., Leeson, A., Li, Y., Bastien, N., Cutts, T., Andonov, A., Cao, J., Booth, T. F., Plummer, F. A., Tyler, S., Baker, L. and Li, X. 2004. Analysis of multimerization of the SARS coronavirus nucleocapsid protein. Biochemical and Biophysical Research Communications, 316(2), 476-483. https://doi.org/10.1016/j.bbrc.2004.02.074
Heo, L., Lee, H. and Seok, C. 2016. GalaxyRefineComplex: Refinement of protein-protein complex model structures driven by interface repacking. Scientific Reports, 6(1), 32153. https://doi.org/10.1038/srep32153
Horton, P., Park, K.J., Obayashi, T., Fujita, N., Harada, H., Adams-Collier, C. J. and Nakai, K. 2007. WoLF PSORT: Protein localization predictor. Nucleic Acids Research, 35(suppl_2), W585-W587. https://doi.org/10.1093/nar/gkm259
Hussain, S. and Gallagher, T. 2010. SARS-coronavirus protein 6 conformations required to impede protein import into the nucleus. Virus Research, 153(2), 299-304. https://doi.org/10.1016/j.virusres.2010.08.017
Iqbal, H. M. N., Romero-Castillo, K. D., Bilal, M. and Parra-Saldivar, R. 2020. The emergence of novel-coronavirus and its replication cycle—An overview. Journal of Pure and Applied Microbiology, 14(1). https://doi.org/10.22207/JPAM.14.1.03
Jiménez-García, B., Pons, C. and Fernández-Recio, J. 2013. pyDockWEB: A web server for rigid-body protein–protein docking using electrostatics and desolvation scoring. Bioinformatics, 29(13), 1698-1699. https://doi.org/10.1093/bioinformatics/btt262
Jumper, J., Evans, R., Pritzel, A., Green, T., Figurnov, M., Ronneberger, O., Tunyasuvunakool, K., Bates, R., Žídek, A., Potapenko, A., Bridgland, A., Meyer, C., Kohl, S. A., Ballard, A. J., Cowie, A., Romera-Paredes, B., Nikolov, S., Jain, R., Adler, J., Back, T., Petersen, S., Reiman, D., Clancy, E., Zielinski, M., Steinegger, M., Pacholska, M., Berghammer, T., Bodenstein, S., Silver, D., Vinyals, O., Senior, A. W., Kavukcuoglu, K., Kohli, P. and Hassabis, D. 2021. Highly accurate protein structure prediction with AlphaFold. Nature, 596(7873), 583-589. https://doi.org/10.1038/s41586-021-03819-2
Kannan, S., Shaik Syed Ali, P., Sheeza, A. and Hemalatha, K. 2020. COVID-19 (Novel Coronavirus 2019)—Recent trends. European Review for Medical and Pharmacological Sciences, 24(4), 2006-2011. https://doi.org/10.26355/eurrev_202002_20378
Kato, K., Ikliptikawati, D. K., Kobayashi, A., Kondo, H., Lim, K., Hazawa, M. and Wong, R. W. 2021. Overexpression of SARS-CoV-2 protein ORF6 dislocates RAE1 and NUP98 from the nuclear pore complex. Biochemical and Biophysical Research Communications, 536, 59-66. https://doi.org/10.1016/j.bbrc.2020.11.115
Kim, S., Thiessen, P. A., Bolton, E. E., Chen, J., Fu, G., Gindulyte, A., Han, L., He, J., He, S., Shoemaker, B. A., Wang, J., Yu, B., Zhang, J. and Bryant, S. H. 2016. PubChem Substance and Compound databases. Nucleic Acids Research, 44(D1), D1202-D1213. https://doi.org/10.1093/nar/gkv951
King, C. R., Tessier, T. M., Dodge, M. J., Weinberg, J. B. and Mymryk, J. S. 2020. Inhibition of Human Adenovirus Replication by the Importin α/β1 Nuclear Import Inhibitor Ivermectin. Journal of Virology, 94(18), e00710-20. https://doi.org/10.1128/JVI.00710-20
Kopecky-Bromberg, S. A., Martínez-Sobrido, L., Frieman, M., Baric, R. A. and Palese, P. 2007. Severe Acute Respiratory Syndrome Coronavirus Open Reading Frame (ORF) 3b, ORF 6, and Nucleocapsid Proteins Function as Interferon Antagonists. Journal of Virology, 81(2), 548-557. https://doi.org/10.1128/JVI.01782-06
Kumar, S., Stecher, G., Li, M., Knyaz, C. and Tamura, K. 2018. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Molecular Biology and Evolution, 35(6), 1547-1549. https://doi.org/10.1093/molbev/msy096
Kyte, J. and Doolittle, R. F. 1982. A simple method for displaying the hydropathic character of a protein. Journal of Molecular Biology, 157(1), 105-132. https://doi.org/10.1016/0022-2836(82)90515-0
Laskowski, R. A., MacArthur, M. W., Moss, D. S. and Thornton, J. M. 1993. PROCHECK: A program to check the stereochemical quality of protein structures. Journal of Applied Crystallography, 26(2), 283-291. https://doi.org/10.1107/S0021889892009944
Li, J.Y., Liao, C.H., Wang, Q., Tan, Y.J., Luo, R., Qiu, Y. and Ge, X.Y. 2020. The ORF6, ORF8 and nucleocapsid proteins of SARS-CoV-2 inhibit type I interferon signaling pathway. Virus Research, 286, 198074. https://doi.org/10.1016/j.virusres.2020.198074
Li, W., Cowley, A., Uludag, M., Gur, T., McWilliam, H., Squizzato, S., Park, Y. M., Buso, N. and Lopez, R. 2015. The EMBL-EBI bioinformatics web and programmatic tools framework. Nucleic Acids Research, 43(W1), W580-W584. https://doi.org/10.1093/nar/gkv279
Liu, D. X., Fung, T. S., Chong, K. K.L., Shukla, A. and Hilgenfeld, R. 2014. Accessory proteins of SARS-CoV and other coronaviruses. Antiviral Research, 109, 97-109. https://doi.org/10.1016/j.antiviral.2014.06.013
Lu, R., Zhao, X., Li, J., Niu, P., Yang, B., Wu, H., Wang, W., Song, H., Huang, B., Zhu, N., Bi, Y., Ma, X., Zhan, F., Wang, L., Hu, T., Zhou, H., Hu, Z., Zhou, W., Zhao, L., Chen, J., Meng, Y., Wang, J., Lin, Y., Yuan, J., Xie, Z., Ma, J., Liu, W. L., Wang, D., Xu, W., Holmes, E. C., Gao, G. F., Wu, G., Chen, W., Shi, W. and Tan, W. 2020. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. The Lancet, 395(10224), 565-574. https://doi.org/10.1016/S0140-6736(20)30251-8
Mastrangelo, E., Pezzullo, M., De Burghgraeve, T., Kaptein, S., Pastorino, B., Dallmeier, K., de Lamballerie, X., Neyts, J., Hanson, A. M., Frick, D. N., Bolognesi, M. and Milani, M. 2012. Ivermectin is a potent inhibitor of flavivirus replication specifically targeting NS3 helicase activity: New prospects for an old drug. Journal of Antimicrobial Chemotherapy, 67(8), 1884-1894. https://doi.org/10.1093/jac/dks147
Mirdita, M., Schütze, K., Moriwaki, Y., Heo, L., Ovchinnikov, S. and Steinegger, M. 2022. ColabFold: Making protein folding accessible to all. Nature Methods, 19(6), 679-682. https://doi.org/10.1038/s41592-022-01488-1
Miyamoto, Y., Itoh, Y., Suzuki, T., Tanaka, T., Sakai, Y., Koido, M., Hata, C., Wang, C. X., Otani, M., Moriishi, K., Tachibana, T., Kamatani, Y., Yoneda, Y., Okamoto, T. and Oka, M. 2022. SARS-CoV-2 ORF6 disrupts nucleocytoplasmic trafficking to advance viral replication. Communications Biology, 5(1), 1-15. https://doi.org/10.1038/s42003-022-03427-4
Narayanan, K., Huang, C. and Makino, S. 2008. SARS coronavirus accessory proteins. Virus Research, 133(1), 113-121. https://doi.org/10.1016/j.virusres.2007.10.009
Ōmura, S. and Crump, A. 2014. Ivermectin: Panacea for resource-poor communities? Trends in Parasitology, 30(9), 445-455. https://doi.org/10.1016/j.pt.2014.07.005
Pallara, C., Jiménez-García, B., Romero, M., Moal, I. H. and Fernández-Recio, J. 2017. pyDock scoring for the new modeling challenges in docking: Protein–peptide, homo-multimers, and domain–domain interactions. Proteins: Structure, Function, and Bioinformatics, 85(3), 487-496. https://doi.org/10.1002/prot.25184
Pettersen, E. F., Goddard, T. D., Huang, C. C., Couch, G. S., Greenblatt, D. M., Meng, E. C. and Ferrin, T. E. 2004. UCSF Chimera—A visualization system for exploratory research and analysis. Journal of Computational Chemistry, 25(13), 1605-1612. https://doi.org/10.1002/jcc.20084
Pumroy, R. A., Ke, S., Hart, D. J., Zachariae, U. and Cingolani, G. 2015. Molecular Determinants for Nuclear Import of Influenza A PB2 by Importin α Isoforms 3 and 7. Structure, 23(2), 374-384. https://doi.org/10.1016/j.str.2014.11.015
Pumroy, R. and Cingolani, G. 2015. Diversification of importin-α isoforms in cellular trafficking and disease states. The Biochemical journal. https://doi.org/10.1042/BJ20141186
Qinfen, Z., Jinming, C., Xiaojun, H., Huanying, Z., Jicheng, H., Ling, F., Kunpeng, L. and Jingqiang, Z. 2004. The life cycle of SARS coronavirus in Vero E6 cells. Journal of Medical Virology, 73(3), 332-337. https://doi.org/10.1002/jmv.20095
Rizzo, E. 2020. Ivermectin, antiviral properties and COVID-19: A possible new mechanism of action. Naunyn-Schmiedeberg’s Archives of Pharmacology, 393(7), 1153-1156. https://doi.org/10.1007/s00210-020-01902-5
Robert, X. and Gouet, P. 2014. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Research, 42(W1), W320-W324. https://doi.org/10.1093/nar/gku316
Rowland, R. R. R., Chauhan, V., Fang, Y., Pekosz, A., Kerrigan, M. and Burton, M. D. 2005. Intracellular Localization of the Severe Acute Respiratory Syndrome Coronavirus Nucleocapsid Protein: Absence of Nucleolar Accumulation during Infection and after Expression as a Recombinant Protein in Vero Cells. Journal of Virology, 79(17), 11507-11512. https://doi.org/10.1128/JVI.79.17.11507-11512.2005
Saha, J. K. and Raihan, Md. J. 2021. The binding mechanism of ivermectin and levosalbutamol with spike protein of SARS-CoV-2. Structural Chemistry, 32(5), 1985-1992. https://doi.org/10.1007/s11224-021-01776-0
Sekimoto, T., Imamoto, N., Nakajima, K., Hirano, T. and Yoneda, Y. 1997. Extracellular signal-dependent nuclear import of Stat1 is mediated by nuclear pore-targeting complex formation with NPI-1, but not Rch1. The EMBO Journal, 16(23), 7067-7077. https://doi.org/10.1093/emboj/16.23.7067
Sen Gupta, P. S., Biswal, S., Panda, S. K., Ray, A. K. and Rana, M. K. 2022. Binding mechanism and structural insights into the identified protein target of COVID-19 and importin-α with in-vitro effective drug ivermectin. Journal of Biomolecular Structure and Dynamics, 40(5), 2217-2226. https://doi.org/10.1080/07391102.2020.1839564
Shereen, M. A., Khan, S., Kazmi, A., Bashir, N. and Siddique, R. 2020. COVID-19 infection: Origin, transmission, and characteristics of human coronaviruses. Journal of Advanced Research, 24, 91-98. https://doi.org/10.1016/j.jare.2020.03.005
Sievers, F. and Higgins, D. G. 2014. Clustal Omega, Accurate Alignment of Very Large Numbers of Sequences. En D. J. Russell (Ed.), Multiple Sequence Alignment Methods (pp. 105-116). Totowa, NJ: Humana Press. https://doi.org/10.1007/978-1-62703-646-7_6
Surjit, M., Liu, B., Jameel, S., Chow, V. T. K. and Lal, S. K. 2004. The SARS coronavirus nucleocapsid protein induces actin reorganization and apoptosis in COS-1 cells in the absence of growth factors. Biochemical Journal, 383(1), 13-18. https://doi.org/10.1042/BJ20040984
Tarendeau, F., Boudet, J., Guilligay, D., Mas, P. J., Bougault, C. M., Boulo, S., Baudin, F., Ruigrok, R. W. H., Daigle, N., Ellenberg, J., Cusack, S., Simorre, J. P. and Hart, D. J. 2007. Structure and nuclear import function of the C-terminal domain of influenza virus polymerase PB2 subunit. Nature Structural & Molecular Biology, 14(3), 229-233. https://doi.org/10.1038/nsmb1212
Timani, K. A., Liao, Q., Ye, L., Zeng, Y., Liu, J., Zheng, Y., Ye, L., Yang, X., Lingbao, K., Gao, J. and Zhu, Y. 2005. Nuclear/nucleolar localization properties of C-terminal nucleocapsid protein of SARS coronavirus. Virus Research, 114(1), 23-34. https://doi.org/10.1016/j.virusres.2005.05.007
Trott, O. and Olson, A. J. 2010. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. Journal of Computational Chemistry, 31(2), 455-461. https://doi.org/10.1002/jcc.21334
Vangone, A., Schaarschmidt, J., Koukos, P., Geng, C., Citro, N., Trellet, M. E., Xue, L. C. and Bonvin, A. M. J. J. 2019. Large-scale prediction of binding affinity in protein–small ligand complexes: The PRODIGY-LIG web server. Bioinformatics, 35(9), 1585-1587. https://doi.org/10.1093/bioinformatics/bty816
Varadi, M., Anyango, S., Deshpande, M., Nair, S., Natassia, C., Yordanova, G., Yuan, D., Stroe, O., Wood, G., Laydon, A., Žídek, A., Green, T., Tunyasuvunakool, K., Petersen, S., Jumper, J., Clancy, E., Green, R., Vora, A., Lutfi, M., Figurnov, M., Cowie, A., Hobbs, N., Kohli, P., Kleywegt, G., Birney, E., Hassabis, D. and Velankar, S. 2022. AlphaFold Protein Structure Database: Massively expanding the structural coverag2e of protein-sequence space with high-accuracy models. Nucleic Acids Research, 50(D1), D439-D444. https://doi.org/10.1093/nar/gkab1061
Wagstaff, K. M., Sivakumaran, H., Heaton, S. M., Harrich, D. and Jans, D. A. 2012. Ivermectin is a specific inhibitor of importin α/β-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus. Biochemical Journal, 443(3), 851-856. https://doi.org/10.1042/BJ20120150
Williams, C. J., Headd, J. J., Moriarty, N. W., Prisant, M. G., Videau, L. L., Deis, L. N., Verma, V., Keedy, D. A., Hintze, B. J., Chen, V. B., Jain, S., Lewis, S. M., Arendall III, W. B., Snoeyink, J., Adams, P. D., Lovell, S. C., Richardson, J. S. and Richardson, D. C. 2018. MolProbity: More and better reference data for improved all-atom structure validation. Protein Science, 27(1), 293-315. https://doi.org/10.1002/pro.3330
Wu, F., Zhao, S., Yu, B., Chen, Y. M., Wang, W., Song, Z. G., Hu, Y., Tao, Z. W., Tian, J. H., Pei, Y. Y., Yuan, M. L., Zhang, Y. L., Dai, F. H., Liu, Y., Wang, Q. M., Zheng, J. J., Xu, L., Holmes, E. C. and Zhang, Y. Z. 2020. A new coronavirus associated with human respiratory disease in China. Nature, 579(7798), 265-269. https://doi.org/10.1038/s41586-020-2008-3
Wulan, W. N., Heydet, D., Walker, E. J., Gahan, M. E. and Ghildyal, R. 2015. Nucleocytoplasmic transport of nucleocapsid proteins of enveloped RNA viruses. Frontiers in Microbiology, 6. https://doi.org/10.3389/fmicb.2015.00553
Wurm, T., Chen, H., Hodgson, T., Britton, P., Brooks, G. and Hiscox, J. A. 2001. Localization to the Nucleolus Is a Common Feature of Coronavirus Nucleoproteins, and the Protein May Disrupt Host Cell Division. Journal of Virology, 75(19), 9345-9356. https://doi.org/10.1128/JVI.75.19.9345-9356.2001
Xu, W., Edwards, M. R., Borek, D. M., Feagins, A. R., Mittal, A., Alinger, J. B., Berry, K. N., Yen, B., Hamilton, J., Brett, T. J., Pappu, R. V., Leung, D. W., Basler, C. F. and Amarasinghe, G. K. 2014. Ebola Virus VP24 Targets a Unique NLS Binding Site on Karyopherin Alpha 5 to Selectively Compete with Nuclear Import of Phosphorylated STAT1. Cell Host & Microbe, 16(2), 187-200. https://doi.org/10.1016/j.chom.2014.07.008
Yang, S. N. Y., Atkinson, S. C., Wang, C., Lee, A., Bogoyevitch, M. A., Borg, N. A. and Jans, D. A. 2020. The broad spectrum antiviral ivermectin targets the host nuclear transport importin α/β1 heterodimer. Antiviral Research, 177, 104760. https://doi.org/10.1016/j.antiviral.2020.104760
Ye, Z., Wong, C. K., Li, P. and Xie, Y. 2008. A SARS-CoV protein, ORF-6, induces caspase-3 mediated, ER stress and JNK-dependent apoptosis. Biochimica et Biophysica Acta (BBA) - General Subjects, 1780(12), 1383-1387. https://doi.org/10.1016/j.bbagen.2008.07.009
Zhao, J., Falcón, A., Zhou, H., Netland, J., Enjuanes, L., Breña, P. P. and Perlman, S. 2009. Severe Acute Respiratory Syndrome Coronavirus Protein 6 Is Required for Optimal Replication. Journal of Virology, 83(5), 2368-2373. https://doi.org/10.1128/JVI.02371-08
Zhou, P., Yang, X. L., Wang, X. G., Hu, B., Zhang, L., Zhang, W., Si, H. R., Zhu, Y., Li, B., Huang, C. L., Chen, H. D., Chen, J. C., Luo, Y., Guo, H., Jiang, R. D., Liu, M. Q., Chen, Y. C., Shen, Z. R., Wang, X., Zheng, X. S., Zhao, K., Chen, Q. J., Deng, F., Liu, L. L., Yan, B., Zhan, F. X., Wang, Y. Y., Xiao, G. F. and Shi, Z. L. 2020. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature, 579(7798), 270-273. https://doi.org/10.1038/s41586-020-2012-7
Descargas
Publicado
Cómo citar
Número
Sección
Licencia
Derechos de autor 2025

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-CompartirIgual 4.0.
La revista Biotecnia se encuentra bajo la licencia Atribución-NoComercial-CompartirIgual 4.0 Internacional (CC BY-NC-SA 4.0)















_(2).jpg)








