Desarrollo de vacunas contra Leishmania mexicana: un enfoque de vacunología reversa
DOI:
https://doi.org/10.18633/biotecnia.v27.2537Palabras clave:
Leishmaniasis cutánea del nuevo mundo, inmunoinformática, diseño de vacunasResumen
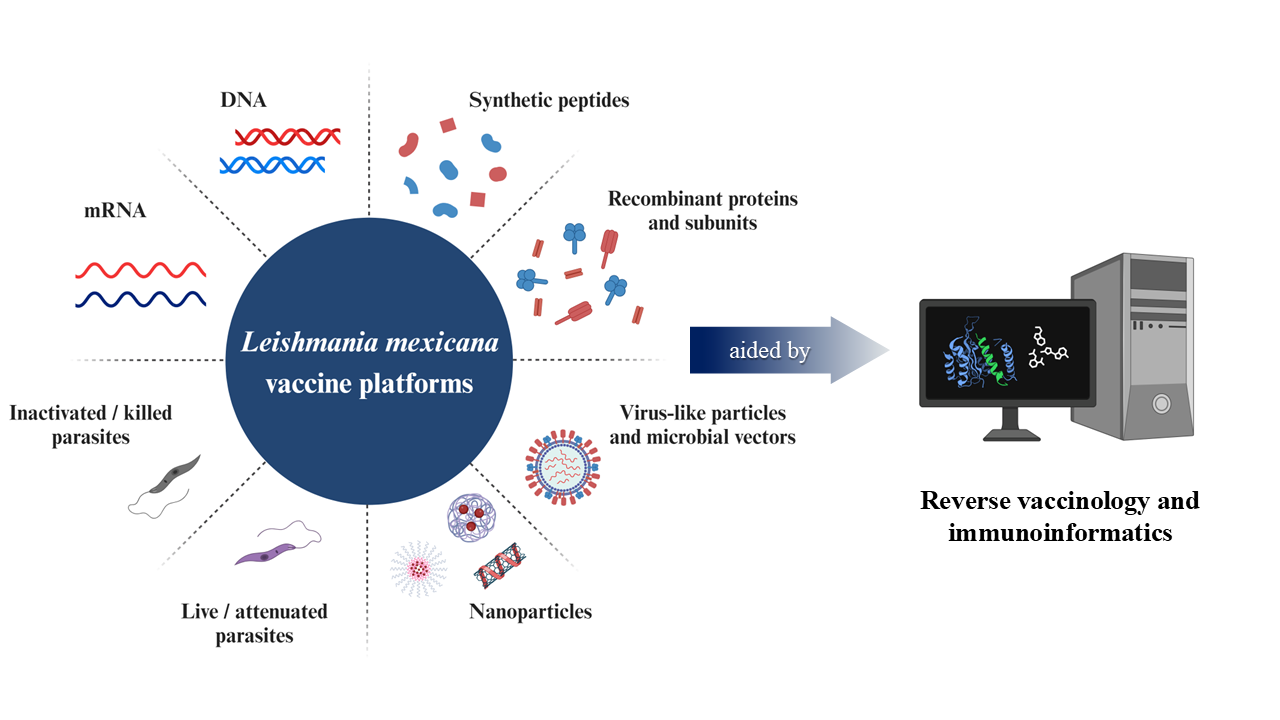
La leishmaniasis cutánea causada por Leishmania mexicana es un importante problema de salud pública en América. En consecuencia, se necesitan enfoques nuevos y más eficaces para controlar la enfermedad. A pesar de los considerables esfuerzos para prevenir y tratar la leishmaniasis cutánea, no se dispone de vacunas licenciadas para humanos, lo que incentiva la investigación en este tema. Los avances en la vacunología reversa y la inmunoinformática han facilitado el diseño de varios candidatos contra la leishmaniasis cutánea. La versatilidad del enfoque de vacunología reversa permite la inclusión de varios epítopos en una sola construcción de vacuna, pudiendo inducir respuestas inmunes protectoras in vivo. Por lo tanto, se espera que el enfoque in silico resuelva los problemas actuales relacionados con la inmunogenicidad, seguridad y costos de producción de las vacunas contra L. mexicana, así como cuestiones relacionadas con la biología del parásito. Este trabajo abarca el estado del arte de las vacunas para L. mexicana, así como las perspectivas y desafíos de la inmunoinformática en la investigación de vacunas contra la leishmaniasis cutánea.
Descargas
Citas
Abadías-Granado, I., Diago, A., Cerro, P.A., Palma-Ruiz, A.M. and Gilaberte, Y. 2021. Cutaneous and Mucocutaneous Leishmaniasis. Actas Dermo-Sifiliograficas 112(7), pp. 601–618. doi: 10.1016/j.ad.2021.02.008.
Aebischer, T., Wolfram, M., Patzer, S.I., Ilg, T., Wiese, M. and Overath, P. 2000. Subunit vaccination of mice against new world cutaneous leishmaniasis: comparison of three proteins expressed in amastigotes and six adjuvants. Infection and immunity 68(3), pp. 1328–1336. Available at: https://pubmed.ncbi.nlm.nih.gov/10678945/
Akbari, M., Oryan, A. and Hatam, G. 2021. Immunotherapy in treatment of leishmaniasis. Immunology letters 233, pp. 80–86. Available at: https://pubmed.ncbi.nlm.nih.gov/33771555/
Akya, A., Farasat, A., Ghadiri, K. and Rostamian, M. 2019. Identification of HLA-I restricted epitopes in six vaccine candidates of Leishmania tropica using immunoinformatics and molecular dynamics simulation approaches. Infection, Genetics and Evolution 75. doi: 10.1016/j.meegid.2019.103953.
Alves-Silva, M.V., Nico, D., De Luca, P.M. and Palatnik De-Sousa, C.B. 2019. The F1F3 Recombinant Chimera of Leishmania donovani-Nucleoside Hydrolase (NH36) and Its Epitopes Induce Cross-Protection Against Leishmania (V.) braziliensis Infection in Mice. Frontiers in immunology 10(APR). Available at: https://pubmed.ncbi.nlm.nih.gov/31024556/
Burgos-Reyes, M.A., Baylón-Pacheco, L., Espíritu-Gordillo, P., Galindo-Gómez, S., Tsutsumi, V. and Rosales-Encina, J.L. 2021. Effect of Prophylactic Vaccination with the Membrane-Bound Acid Phosphatase Gene of Leishmania mexicana in the Murine Model of Localized Cutaneous Leishmaniasis. Journal of Immunology Research 2021. doi: 10.1155/2021/6624246.
Calzetta, L. et al., 2020. Immunoprophylaxis pharmacotherapy against canine leishmaniosis: A systematic review and meta-analysis on the efficacy of vaccines approved in European Union. Vaccine 38(43), pp. 6695–6703. Available at: https://pubmed.ncbi.nlm.nih.gov/32883556/
Cecílio, P., Oristian, J., Meneses, C., Serafim, T.D., Valenzuela, J.G., Cordeiro da Silva, A. and Oliveira, F. 2020. Engineering a vector-based pan-Leishmania vaccine for humans: proof of principle. Scientific reports 10(1). Available at: https://pubmed.ncbi.nlm.nih.gov/33122717/
Cianci, R. and Franza, L. 2022. Recent Advances in Vaccine Technology and Design. Vaccines 10(4). doi: 10.3390/vaccines10040624.
Coler, R.N. and Reed, S.G. 2005. Second-generation vaccines against leishmaniasis. Trends in parasitology 21(5), pp. 244–249. Available at: https://pubmed.ncbi.nlm.nih.gov/15837614/
Convit, J., Ulrich, M., Polegre, M.A., Avila, A., Rodríguez, N., Mazzedo, M.I. and Blanco, B. 2004. Therapy of Venezuelan patients with severe mucocutaneous or early lesions of diffuse cutaneous leishmaniasis with a vaccine containing pasteurized Leishmania promastigotes and bacillus Calmette-Guerin: preliminary report. Memorias do Instituto Oswaldo Cruz 99(1), pp. 57–62. Available at: https://pubmed.ncbi.nlm.nih.gov/15057348/
Daneshvar, H., Hagan, P. and Phillips, R.S. 2003. Leishmania mexicana H-line attenuated under pressure of gentamicin, potentiates a Th1 response and control of cutaneous leishmaniasis in BALB/c mice. Parasite immunology 25(11–12), pp. 589–596. Available at: https://pubmed.ncbi.nlm.nih.gov/15053780/
Dhanda, S.K., Vir, P. and Raghava, G.P.S. 2013. Designing of interferon-gamma inducing MHC class-II binders. Biology Direct 8(1), p. 30. Available at: /pmc/articles/PMC4235049/
Dinc, R. 2022. Leishmania Vaccines: the Current Situation with Its Promising Aspect for the Future. Korean Journal of Parasitology 60(6), pp. 379–391. doi: 10.3347/kjp.2022.60.6.379.
Dumonteil, E., Jesus, R.S.M., Javier, E.O. and Del Rosario, G.M.M. 2003. DNA vaccines induce partial protection against Leishmania mexicana. Vaccine 21(17–18), pp. 2161–2168. doi: 10.1016/S0264-410X(02)00769-7.
Flórez, M.M., Rodríguez, R., Cabrera, J.A., Robledo, S.M. and Delgado, G. 2021. Leishmania spp Epitopes in Humans Naturally Resistant to the Disease: Working Toward a Synthetic Vaccine. Frontiers in Cellular and Infection Microbiology 11. doi: 10.3389/fcimb.2021.631019.
González, C.R. et al., 1998. Immunogenicity of a Salmonella typhi CVD 908 candidate vaccine strain expressing the major surface protein gp63 of Leishmania mexicana mexicana. Vaccine 16(9–10), pp. 1043–1052. Available at: https://pubmed.ncbi.nlm.nih.gov/9682357/
Gonzalez-Galarza, F.F. et al., 2020. Allele frequency net database (AFND) 2020 update: gold-standard data classification, open access genotype data and new query tools. Nucleic Acids Research 48(D1), pp. D783–D788. Available at: https://academic.oup.com/nar/article/48/D1/D783/5624967
Graña, C. et al., 2022. Efficacy and safety of COVID-19 vaccines. The Cochrane database of systematic reviews 12(12). Available at: https://pubmed.ncbi.nlm.nih.gov/36473651/
Gupta, O., Pradhan, T., Bhatia, R. and Monga, V. 2021. Recent advancements in anti-leishmanial research: Synthetic strategies and structural activity relationships. European Journal of Medicinal Chemistry 223. doi: 10.1016/j.ejmech.2021.113606.
Herrera, G. et al., 2020. An interactive database of Leishmania species distribution in the Americas. Scientific Data 7(1). doi: 10.1038/s41597-020-0451-5.
Hwang, W., Lei, W., Katritsis, N.M., MacMahon, M., Chapman, K. and Han, N. 2021. Current and prospective computational approaches and challenges for developing COVID-19 vaccines. Advanced Drug Delivery Reviews 172, pp. 249–274. doi: 10.1016/j.addr.2021.02.004.
Lee, C., Su, B.H. and Tseng, Y.J. 2022. Comparative studies of AlphaFold, RoseTTAFold and Modeller: A case study involving the use of G-protein-coupled receptors. Briefings in Bioinformatics 23(5). doi: 10.1093/bib/bbac308.
Martinelli, D.D. 2022. in silico vaccine design: A tutorial in immunoinformatics. Healthcare Analytics 2. doi: 10.1016/j.health.2022.100044.
Moafi, M., Sherkat, R., Taleban, R. and Rezvan, H. 2019. Leishmania Vaccines Entered in Clinical Trials: A Review of Literature. International journal of preventive medicine 10(1), pp. 1–6. Available at: https://pubmed.ncbi.nlm.nih.gov/31360342/
Moreira, P.O.L., Nogueira, P.M. and Monte-Neto, R.L. 2023. Next-Generation Leishmanization: Revisiting Molecular Targets for Selecting Genetically Engineered Live-Attenuated Leishmania. Microorganisms 2023, Vol. 11, Page 1043 11(4), p. 1043. Available at: https://www.mdpi.com/2076-2607/11/4/1043/htm
Motamedpour, L., Dalimi, A., Pirestani, M. and Ghaffarifar, F. 2020. in silico analysis and expression of a new chimeric antigen as a vaccine candidate against cutaneous leishmaniasis. Iranian Journal of Basic Medical Sciences 23(11), pp. 1409–1418. doi: 10.22038/ijbms.2020.45394.10561.
Moxon, R., Reche, P.A. and Rappuoli, R. 2019. Editorial: Reverse Vaccinology. Frontiers in Immunology 10, p. 2776. Available at: /pmc/articles/PMC6901788/
PAHO. 2020. Leishmaniasis: Epidemiological Report of the Americas.
Pinart, M. et al., 2020. Interventions for American cutaneous and mucocutaneous leishmaniasis. Cochrane Database of Systematic Reviews 2020(8). doi: 10.1002/14651858.CD004834.PUB3.
Rafati, S., Shadab, M., Didwania, N., Sabur, A. and Ali, N. 2017. Alternative to Chemotherapy—The Unmet Demand against Leishmaniasis. Immunol 8, p. 1779. Available at: www.frontiersin.org
Rapin, N., Lund, O., Bernaschi, M. and Castiglione, F. 2010. Computational Immunology Meets Bioinformatics: The Use of Prediction Tools for Molecular Binding in the Simulation of the Immune System. PLOS ONE 5(4), p. e9862. Available at: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0009862
Rappuoli, R. 2000. Reverse vaccinology. Current opinion in microbiology 3(5), pp. 445–450. Available at: https://pubmed.ncbi.nlm.nih.gov/11050440/
Rawal, K. et al., 2021. Identification of vaccine targets in pathogens and design of a vaccine using computational approaches. Scientific Reports 2021 11:1 11(1), pp. 1–25. Available at: https://www.nature.com/articles/s41598-021-96863-x c
Rogers, M.B. et al., 2011. Chromosome and gene copy number variation allow major structural change between species and strains of Leishmania. Genome Research 21(12), pp. 2129–2142. doi: 10.1101/GR.122945.111.
Rosado-Vallado, M., Mut-Martin, M., Del Rosario García-Miss, M. and Dumonteil, E. 2005. Aluminium phosphate potentiates the efficacy of DNA vaccines against Leishmania mexicana. Vaccine 23(46–47), pp. 5372–5379. Available at: https://pubmed.ncbi.nlm.nih.gov/16054271/
Saravia, N.G. et al., 2006. Pathogenicity and protective immunogenicity of cysteine proteinase-deficient mutants of Leishmania mexicana in non-murine models. Vaccine 24(19), pp. 4247–4259. Available at: https://pubmed.ncbi.nlm.nih.gov/16216395/
Shams, M. et al., [no date]. Engineering a multi-epitope vaccine candidate against Leishmania infantum using comprehensive Immunoinformatics methods. Biologia 1, p. 3. Available at: https://doi.org/10.1007/s11756-021-00934-3
Shams, M., Nourmohammadi, H., Majidiani, H., Shariatzadeh, S.A., Asghari, A., Fatollahzadeh, M. and Irannejad, H. 2022. Engineering a multi-epitope vaccine candidate against Leishmania infantum using comprehensive Immunoinformatics methods. Biologia 77(1), pp. 277–289. doi: 10.1007/s11756-021-00934-3.
Singh, G., Pritam, M., Banerjee, M., Singh, A.K. and Singh, S.P. 2020. Designing of precise vaccine construct against visceral leishmaniasis through predicted epitope ensemble: A contemporary approach. Computational Biology and Chemistry 86. doi: 10.1016/j.compbiolchem.2020.107259.
Tahamtan, A., Charostad, J., Javad, S., Shokouh, H. and Barati, M. 2017. An Overview of History, Evolution, and Manufacturing of Various Generations of Vaccines. Journal of Archives in Military Medicine 2017 5:3 5(3), p. 12315. Available at: https://brieflands.com/articles/jamm-12315.html
Tejeda-Mansir, A., García-Rendón, A. and Guerrero-Germán, P. 2019. Plasmid-DNA lipid and polymeric nanovaccines: a new strategic in vaccines development. Biotechnology & genetic engineering reviews 35(1), pp. 46–68. Available at: https://pubmed.ncbi.nlm.nih.gov/30587085/
Vivona, S., Gardy, J.L., Ramachandran, S., Brinkman, F.S.L., Raghava, G.P.S., Flower, D.R. and Filippini, F. 2008. Computer-aided biotechnology: from immuno-informatics to reverse vaccinology. Trends in Biotechnology 26(4), pp. 190–200. doi: 10.1016/j.tibtech.2007.12.006.
Volpedo, G. et al., 2022. Centrin-deficient Leishmania mexicana confers protection against New World cutaneous leishmaniasis. NPJ vaccines 7(1). Available at: https://pubmed.ncbi.nlm.nih.gov/35236861/
de Vries, H.J.C. and Schallig, H.D. 2022. Cutaneous Leishmaniasis: A 2022 Updated Narrative Review into Diagnosis and Management Developments. American Journal of Clinical Dermatology 23(6), pp. 823–840. doi: 10.1007/s40257-022-00726-8.
Wheeler, R.J. 2021. A resource for improved predictions of Trypanosoma and Leishmania protein three-dimensional structure. PLoS ONE 16(11 November). doi: 10.1371/journal.pone.0259871.
Woolums, A.R. and Swiderski, C. 2021. New approaches to vaccinology made possible by advances in next generation sequencing, bioinformatics and protein modeling. Current Issues in Molecular Biology 42, pp. 605–634. doi: 10.21775/cimb.042.605.
Zabala-Peñafiel, A., Todd, D., Daneshvar, H. and Burchmore, R. 2020. The potential of live attenuated vaccines against Cutaneous Leishmaniasis. Experimental Parasitology 210, p. 107849. doi: 10.1016/J.EXPPARA.2020.107849.
Descargas
Publicado
Cómo citar
Número
Sección
Licencia
Derechos de autor 2025

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-CompartirIgual 4.0.
La revista Biotecnia se encuentra bajo la licencia Atribución-NoComercial-CompartirIgual 4.0 Internacional (CC BY-NC-SA 4.0)















_(2).jpg)








