Evaluación de un medio mínimo a base de nopal para el crecimiento de Lactobacillus spp.
DOI:
https://doi.org/10.18633/biotecnia.v27.2550Palabras clave:
Lactobacillus spp, Nopal silvestre, O. robusta, medio mínimoResumen
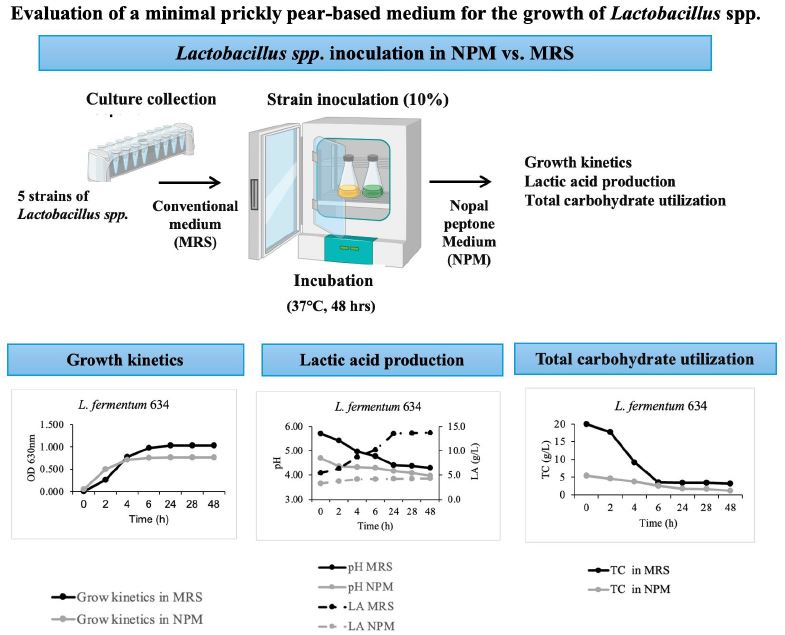
El objetivo de este estudio fue evaluar el potencial del nopal silvestre deshidratado (Opuntia robusta) para el crecimiento de Lactobacillus spp. Se formuló un medio mínimo a base de nopal y peptona (NPM) el cual fue inoculado por separado con las cinco cepas de Lactobacillus. Se realizaron cinéticas en las cuales se determinaron el crecimiento, el consumo de azúcares totales, el comportamiento del pH y la producción de ácido láctico, comparando con el medio de cultivo comercial (MRS). Los resultados muestran que el medio NPM proporciona los sustratos mínimos necesarios para el crecimiento de las cepas evaluadas, siendo L. fermentum 634 la más eficiente, alcanzando una OD630 de 0.7667 ± 0.0527 y 1.0639 ± 0.0616 en NPM y MRS respectivamente. Notablemente, no se observó fase de latencia (λ = 0) en NPM, lo que sugiere una rápida adaptación bacteriana, mientras que en MRS L. fermentum 634 presentó una fase de latencia de 1.137 h. El tiempo de generación en NPM (2.67 h) fue comparable al de MRS (2.31 h), lo que indica una replicación bacteriana eficiente. Estos resultados sugieren que el nopal puede utilizarse como base para la formulación de medios de cultivo, los cuales pueden mejorarse añadiendo sustratos específicos requeridos por las bacterias, con el fin de optimizar procesos de fermentación industrial sostenibles y económicos.
Descargas
Citas
Abdel-Rahman, M.A., Tashiro, Y. and Sonomoto, K. (2013) Recent advances in lactic
acid production by microbial fermentation processes. Biotechnology Advances, 31(6), pp. 877–902. Available at: https://doi.org/10.1016/j.biotechadv.2013.04.002.
Astudillo, Á., Rubilar, O., Briceño, G., Diez, M.C. and Schalchli, H. (2023) Advances in
agroindustrial waste as a substrate for obtaining eco-friendly microbial products. Sustainability, 15(4), p. 3467. Available at: https://doi.org/10.3390/su15043467.
Axelsson, L. (1998) Lactic acid bacteria: Classification and physiology. In: Salminen, S.
and Wright, A.V. (eds.) Lactic Acid Bacteria: Microbiology and Functional Aspects. 2nd ed. New York: Marcel Dekker, pp. 1–72.
Azad, M.A., Sarker, M., Li, T. and Yin, J. (2018) Probiotic species in the modulation of
gut microbiota: An overview. BioMed Research International, 2018, Article ID
Available at: https://doi.org/10.1155/2018/9478630.
Beltrán-Orozco, M.A., Romero-Nava, R., Díaz-Galindo, E. and González-Chávez, M.M.
(2021) Traditional and emerging uses of Opuntia ficus-indica in human health and nutrition. Journal of Ethnopharmacology, 267, p. 113476. Available at: https://doi.org/10.1016/j.jep.2020.113476.
Bernardeau, M., Guéguen, M. and Vernoux, J.P. (2006) Beneficial lactobacilli in food
and feed: Long-term use, biodiversity and proposals for specific and realistic safety assessments. FEMS Microbiology Reviews, 30(4), pp. 487–513. Available at: https://doi.org/10.1111/j.1574-6976.2006.00020.x.
Bouteille, R., Gaudet, M., Lecanu, B. and This, H. (2013) Monitoring lactic acid
production during milk fermentation by in situ quantitative proton nuclear magnetic resonance spectroscopy. Journal of Dairy Science, 96(4), pp. 2071–2080. Available at: https://doi.org/10.3168/jds.2012-6092.
Cheigh, C.I., Choi, H.J., Park, H., Kim, S.B., Kook, M.C., Kim, T.S., Hwang, J.K. and Pyun,
Y.R. (2002) ‘Influence of growth conditions on the production of a nisin-like bacteriocin by Lacto-coccus lactis subsp. lactis A164 isolated from kimchi’, Journal of Biotechnology, 95(3), pp. 225–235. Available at: https://doi.org/10.1016/S0168-1656(02)00010-X.
Claesson, M.J., van Sinderen, D. and O’Toole, P.W. (2007) The genus Lactobacillus—a
genomic basis for understanding its diversity. FEMS Microbiology Letters, 292(1), pp. 2–12. Available at: https://doi.org/10.1111/j.1574-6968.2006.00596.x.
Coda, R., Rizzello, C.G., Di Cagno, R., et al. (2011) Antifungal activity of Lactobacillus
plantarum and other lactobacilli isolated from sourdough: Identification of a novel antifungal pep-tide. Food Microbiology, 28(1), pp. 161–166. Available at: https://doi.org/10.1016/j.fm.2010.06.003.
Derabli, M., Martínez-Ávila, G.C., Romero-Carrillo, E., et al. (2022) Extraction and
characterization of bioactive compounds from nopal (Opuntia ficus-indica) cladodes: Application in functional foods and health. Plant Foods for Human Nutrition, 77(4), pp. 425–435. Available at: https://doi.org/10.1007/s11130-022-00976-y.
Frati, A.C., Jiménez, E. and Ariza, C.R. (2021) Hypoglycemic effect of Opuntia ficus
indica in non-insulin-dependent diabetes mellitus patients. Diabetes Care, 44(1), pp. 455–456. Available at: https://doi.org/10.2337/diacare.44.1.455.
Feugang, J.M., Konarski, P., Zou, D., Stintzing, F.C. and Carle, R. (2018) Cactus stem
(Opuntia spp.) as a functional food. Frontiers in Bioscience (Scholar Edition), 10(2), pp. 473–480. Available at: https://doi.org/10.2741/S570.
Gao, X., Qiao, S.Y. and Lu, W.Q. (2009) Determination of an economical medium for
growth of Lactobacillus fermentum using response surface methodology. Letters in Applied Micro-biology, 49(5), pp. 556–561. Available at: https://doi.org/10.1111/j.1472-765X.2009.02705.x.
Gänzle, M.G. (2014) Enzymatic and bacterial conversions during sourdough
fermentation. Food Microbiology, 37, pp. 2–10. Available at: https://doi.org/10.1016/j.fm.2013.04.007.
Gallego, M.G., Arteaga, G.A. and Martínez, B. (2020) Nutritional evaluation of fermented
dairy and cereal products. Food Chemistry, 308, p. 125664. Available at: https://doi.org/10.1016/j.foodchem.2019.125664.
García, M., et al. (2023) Optimisation of lactic acid production using cost-effective
agro-industrial residues. Faraday Discussions, 245, pp. 213–229. Available at: https://doi.org/10.1039/D3FD00013F.
García-Cayuela, T., Gómez de Cadiñanos, L.P., Peláez, C. and Requena, T. (2014)
Nutritional improvement of an amaranth-based fermented beverage by Lactobacillus reuteri using response surface methodology. Food Research International, 62, pp. 1045–1051. Available at: https://doi.org/10.1016/j.foodres.2014.05.053.
Ginestra, G., Parker, M.L., Bennett, R.N., et al. (2009) Anatomical, chemical, and
biochemical characterization of cladodes from prickly pear (Opuntia ficus-indica (L.) Mill.). Journal of Agricultural and Food Chemistry, 57(21), pp. 10323–10330. Available at: https://doi.org/10.1021/jf901798h.
Goh, Y.J. and Klaenhammer, T.R. (2013) Genomics of stress response in lactic acid
bacteria. Microbial Cell Factories, 12(Suppl 1), p. S7. Available at: https://doi.org/10.1186/1475-2859-12-S1-S7.
Guevara-Arauza, J.C., Pacheco, S. and Santiago, P. (2020) Characterization of prickly
pear (Opuntia ficus-indica) extract as a potential low-cost culture medium for lactic acid bacteria. Revista Fitotecnia Mexicana, 43(2), pp. 107–114. Available at: https://doi.org/10.35196/rfm.2020.2.
Hammes, W.P. and Hertel, C. (2006) The genera Lactobacillus and Carnobacterium. In:
The Prokaryotes. 3rd ed. New York: Springer, pp. 320–403. Available at: https://doi.org/10.1007/0-387-30744-3_13.
Hayek, S.A., Gyawali, R., Aljaloud, S.O., Krastanov, A. and Ibrahim, S.A. (2019)
Cultivation media for lactic acid bacteria used in dairy products. Journal of Dairy Research, 86(4), pp. 490–502. Available at: https://doi.org/10.1017/S002202991900075X.
Hernández-Urbiola, M.I., Pérez-Torrero, E. and Rodríguez-García, M.E. (2019) Chemical
analysis of nutritional content of prickly pads (Opuntia ficus-indica) at varied ages in an organic harvest. International Journal of Environmental Research and Public Health, 16(5), p. 897. Availa-ble at: https://doi.org/10.3390/ijerph16050897.
Hernández-Urbiola, M.I., Contreras-Padilla, M., Pérez-Torrero, E., Hernández-Quevedo, G.,
Rojas-Molina, J.I., Cortes, M.E. and Rodríguez-García, M.E. (2010) ‘Study of nutritional composition of nopal (Opuntia ficus-indica cv. Redonda) at different maturity stages’, The Open Nutrition Journal, 4, pp. 11–16. Available at: https://doi.org/10.2174/1874288201004010011.
Hébert, E.M., Raya, R.R. and de Giori, G.S. (2004) Nutritional requirements and nitrogen
dependent regulation of proteinase activity in Lactobacillus delbrueckii subsp. lactis. Applied and Environmental Microbiology, 70(3), pp. 1239–1244. Available at: https://doi.org/10.1128/AEM.70.3.1239-1244.2004.
Hill, C., Guarner, F., Reid, G., Gibson, G.R., Merenstein, D.J., Pot, B., et al. (2014) Expert
consensus document: The International Scientific Association for Probiotics and Prebiotics consen-sus statement on the scope and appropriate use of the term probiotic. Nature Reviews Gastroenter-ology & Hepatology, 11(8), pp. 506–514. Available at: https://doi.org/10.1038/nrgastro.2014.66.
Hodge, J.E. and Hofreiter, B.T. (1962) Determination of reducing sugars and
carbohydrates. In: Whistler, R.L. and Wolfrom, M.L. (eds.) Methods in Carbohydrate Chemistry. Vol. 1. New York: Academic Press, p. 380–394.
Inglese, P., Basile, F. and Schirra, M. (2002) Cactus pear fruit production. In Cactaceae:
Biology and Uses (pp. 163–183). University of California Press. Available at: https://doi.org/10.1525/california/9780520231573.003.0010.
Ivey, M., Massel, M. and Phister, T.G. (2013) Microbial interactions in food
fermentations. Comprehensive Reviews in Food Science and Food Safety, 4, pp. 141–162.
Karlsson, F.H., Ussery, D.W., Nielsen, J. and Nookaew, I. (2011) A closer look at
Bacteroides: Phylogenetic relationship and genomic implications of a life in the human gut. Microbi-al Ecology, 61(3), pp. 473–485. Available at: https://doi.org/10.1007/s00248-010-9796-1.
Laitila, A., Katina, K. and Mattila-Sandholm, T. (2020) Fermentation of cereals using
Lactobacillus strains. Food Microbiology, 86, p. 103309. Available at: https://doi.org/10.1016/j.fm.2019.103309.
LeBlanc, J.G., Milani, C., de Giori, G.S., Sesma, F., van Sinderen, D. and Ventura, M.
(2013) Bacteria as vitamin suppliers to their host: A gut microbiota perspective. Current Opinion in Biotechnology, 24(2), pp. 160–168. Available at: https://doi.org/10.1016/j.copbio.2012.08.005.
Lee, E., Jung, S.R., Lee, S.Y., Lee, N.K., Paik, H.D. and Lim, S.I. (2018) Lactobacillus
plantarum strain Ln4 attenuates diet-induced obesity, insulin resistance, and changes in hepatic mRNA levels associated with glucose and lipid metabolism. Nutrients, 10(5), p. 643. Available at: https://doi.org/10.3390/nu10050643.
Le Rouzic, M., Bruniaux, P., Raveschot, C., Krier, F., Phalip, V., Ravallec, R., Cudennec,
B. and Coutte, F. (2023) Lactobacillus use for plant fermentation: New ways for plant-based prod-uct valorization. In: Lactobacillus – A Multifunctional Genus. IntechOpen. Available at: https://doi.org/10.5772/intechopen.104958.
Liu, S.Q. (2003) Practical implications of lactate and pyruvate metabolism by lactic acid
bacteria in food and beverage fermentations. International Journal of Food Microbiology, 83(2), pp. 115–131. Available at: https://doi.org/10.1016/s0168-1605(02)00366-5.
Marco, M.L., Heeney, D., Binda, S., Cifelli, C.J., Cotter, P.D., Foligné, B., Gänzle, M., et al.
(2017) Health benefits of fermented foods: microbiota and beyond. Current Opinion in Biotechnol-ogy, 44, pp. 94–102. Available at: https://doi.org/10.1016/j.copbio.2016.11.010.
Monrroy, M., García, E., Ríos, K. and García, J.R. (2017) Extraction and physicochemical
characterization of mucilage from Opuntia cochenillifera (L.) Miller. Journal of Chemistry, 2017, Article ID 4301901. Available at: https://doi.org/10.1155/2017/4301901.
Morales, P., Ramírez-Moreno, E., Sánchez-Mata, M.C. and Fernández-Ruiz, V. (2019)
Opuntia spp.: Health benefits due to its bioactive compounds and its industrial utility. Current Pharmaceutical Design, 25(16), pp. 1723–1731. Available at: https://doi.org/10.2174/1381612825666190718113831.
Moreno-Alvarez, M.J., Martínez-Sánchez, C.E. and Sáenz-Hernández, C. (2000)
Physicochemical composition of nopal pads (Opuntia spp.) at different stages of maturity. Journal of the Professional Association for Cactus Development, 4, pp. 35–45.
Nev, O.A., Lindsay, R.J., Jepson, A., Butt, L., Beardmore, R.E. and Gudelj, I. (2023)
Predicting microbial growth dynamics in response to nutrient availability. PLoS Computational Bi-ology, 19(6), p. e1008945. Available at: https://doi.org/10.1371/journal.pcbi.1008945.
Pimienta-Barrios, E. (1994) Nopalitos production, processing and marketing. Journal of the
Professional Association for Cactus Development, 1, pp. 58–78.
Plaza-Díaz, J., Ruiz-Ojeda, F.J., Vilchez-Padial, L.M. and Gil, Á. (2017) Evidence of the
anti-inflammatory effects of probiotics and synbiotics in intestinal chronic diseases. Nutrients, 9(6), p. 1–20. Available at: https://doi.org/10.3390/nu9060555.
Reddy, G., Altaf, M., Naveena, B.J., Venkateshwar, M. and Kumar, E.V. (2008) Amylolytic
bacterial lactic acid fermentation—A review. Biotechnology Advances, 26(1), pp. 22–34. Available at: https://doi.org/10.1016/j.biotechadv.2007.07.004.
Sáenz, C., Sepúlveda, E. and Matsuhira, B. (2004) Opuntia spp. mucilage’s: A functional
component with industrial perspectives. Journal of Arid Environments, 57(3), pp. 275–290. Available at: https://doi.org/10.1016/S0140-1963(03)00106-X.
Sáenz-Hernández, C., Corrales-García, J., Aquino-Bolaños, T. and De La Rosa, A.P.B. (2020)
Nopalitos, mucilage, fiber, and cochineal. In Nopal (Opuntia spp.) (pp. 59–104). Springer, Cham. Available at: https://doi.org/10.1007/978-3-319-05398-9_4.
Sanders, M.E., Merenstein, D.J., Reid, G., Gibson, G.R. and Rastall, R.A. (2019) Probiotics
and prebiotics in intestinal health and disease: From biology to the clinic. Nature Reviews Gastroen-terology & Hepatology, 16(10), pp. 605–616. Available at: https://doi.org/10.1038/s41575-019-0173-3.
Santos, A.C., Silva, N.S., Ramos, C.L. and Schwan, R.F. (2021) Selection of wild lactic acid
bacteria isolated from cocoa fermentations with probiotic potential and in vitro inhibition of Clos-tridium difficile and Escherichia coli pathogens. Food Research International, 140, p. 109856. Available at: https://doi.org/10.1016/j.foodres.2020.109856.
Seale, R.B., Millar, B.C. and Moore, J.E. (2006) Bacterial growth dynamics in defined and
complex media: Time course in batch culture. Journal of Applied Microbiology, 100(5), pp. 1074–1080. Available at: https://doi.org/10.1111/j.1365-2672.2006.02871.x.
Shah, N.P. (2007) Functional cultures and health benefits. International Dairy Journal,
(11), pp. 1262–1277. Available at: https://doi.org/10.1016/j.idairyj.2007.01.014
Ślizewska, K. and Chlebicz-Wójcik, A. (2020) Growth kinetics of probiotic Lactobacillus
strains in the alternative, cost-efficient semi-solid fermentation medium. Biology (Basel), 9(12), p. 423. Available at: https://doi.org/10.3390/biology9120423.
Stintzing, F.C. and Carle, R. (2005) Cactus stems (Opuntia spp.): A review on their chemistry,
technology, and uses. Molecular Nutrition & Food Research, 49(2), pp. 175–194. Available at: https://doi.org/10.1002/mnfr.200400071.
Zwietering, M.H., Jongenburger, I., Rombouts, F.M. and Van’t Riet, K. (1990) Modeling of
the bacterial growth curve. Applied and Environmental Microbiology, 56, pp. 1875–1881. Available at: https://doi.org/10.1128/aem.56.6.1875-1881.1990.
Szczerbiec, D., Piechocka, J., Głowacki, R. and Torzewska, A. (2022) Organic acids secreted
by Lactobacillus spp. isolated from urine and their antimicrobial activity against uropathogenic Pro-teus mirabilis. Molecules, 27(17), p. 5557. Available at: https://doi.org/10.3390/molecules27175557.
Teusink, B. and Smid, E.J. (2006) Modelling strategies for the industrial exploitation of lactic
acid bacteria. Nature Reviews Microbiology, 4(1), pp. 46–56. Available at: https://doi.org/10.1038/nrmicro1301.
Turroni, F., Ventura, M., Buttó, L.F., Duranti, S., O’Toole, P.W., Motherway, M.O. and van
Sinderen, D. (2014) Molecular dialogue between the human gut microbiota and the host: A Lacto-bacillus and Bifidobacterium perspective. Cellular and Molecular Life Sciences, 71(2), pp. 183–203. Available at: https://doi.org/10.1007/s00018-013-1318-0.
Van der Meulen, R., Avonts, L. and De Vuyst, L. (2004) Growth of Lactobacillus species in
different types of medium and their degradation of fructo-oligosaccharides. Applied Microbiology and Biotechnology, 64(2), pp. 314–321. Available at: https://doi.org/10.1007/s00253-003-1455-0.
Verni, M., Rizzello, C.G. and Coda, R. (2020) Fermentation biotechnology applied to cereal
industry by-products: Nutritional and functional insights. Frontiers in Nutrition, 7, p. 42. Available at: https://doi.org/10.3389/fnut.2020.00042.
Zárate, G., Chaia, A.P., González, S. and Oliver, G. (2002) Viability and biological activity
of probiotic bacteria in bovine milk and fermented dairy products. International Dairy Journal, 12(4), pp. 217–224. Available at: https://doi.org/10.1016/S0958-6946(01)00135-5.
Zhang, J., Bu, Y., Zhang, C., Yi, H., Liu, D. and Jiao, J. (2020) Development of a low-cost
and high-efficiency culture medium for bacteriocin Lac-B23 production by Lactobacillus plantarum J23. Biology, 9(7), p. 171. Available at: https://doi.org/10.3390/biology9070171.
Zhao, J., Liu, P. and Zhang, H. (2021) Fermentation of cereals and legumes by lactic acid
bacteria. Frontiers in Microbiology, 12, p. 635864. Available at: https://doi.org/10.3389/fmicb.2021.635864.
Zheng, J., Wittouck, S., Salvetti, E., et al. (2020) A taxonomic note on the genus
Lactobacillus. International Journal of Systematic and Evolutionary Microbiology, 70(4), pp. 2782–2858. Available at: https://doi.org/10.1099/ijsem.0.004107.
Descargas
Publicado
Cómo citar
Número
Sección
Licencia
Derechos de autor 2025

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-CompartirIgual 4.0.
La revista Biotecnia se encuentra bajo la licencia Atribución-NoComercial-CompartirIgual 4.0 Internacional (CC BY-NC-SA 4.0)















_(2).jpg)








