proteína Alfa-1 antitripsina y su papel en la fisiopatología del cáncer
DOI:
https://doi.org/10.18633/biotecnia.v26.2287Palabras clave:
Antiproteasa, Progresión tumoral, Inflamación, Matriz extracelular, Respuesta inmuneResumen
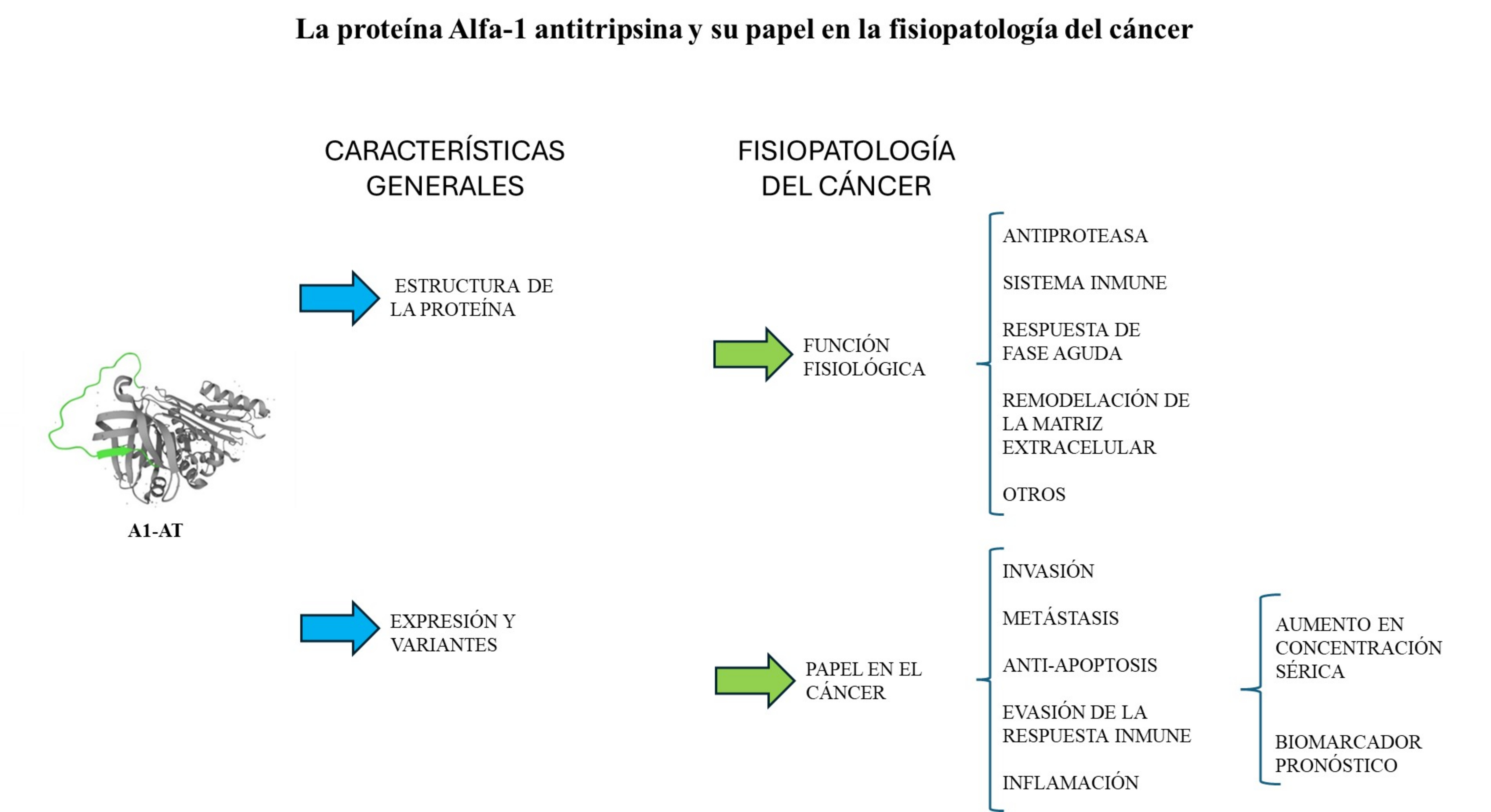
La proteína α1-AT posee una amplia gama de funciones biológicas, su función principal es proteger al pulmón contra las elastasas producidas por los neutrófilos. Sin embargo, también está relacionada con diferentes procesos patológicos, como el cáncer. Entre los tipos de cáncer a los que se ha asociado se encuentra cáncer de mama, próstata, pulmón, cuello uterino, vejiga y colorrectal, entre otros. Asimismo, diferentes estudios han reportado concentraciones aumentadas en los pacientes con cáncer en comparación con sujetos control. Además, la proteína α1-AT se ha asociado como un posible biomarcador en diferentes tipos de cáncer y se ha relacionado con la progresión tumoral. Actualmente, los mecanismos fisiopatológicos y moleculares de la α1-AT en el cáncer aún no son claros. Sin embargo, podría estar participando en diferentes procesos biológicos y moleculares en el microambiente tumoral, lo que podría ser una causa del aumento de la concentración sistémica. En conclusión, el presente trabajo se enfoca en describir la estructura de la α1-AT y recopilar sus funciones más relevantes en procesos fisiológicos y patológicos, como el cáncer.
Descargas
Citas
Aldecoa, F. and Ávila, J. (2021). La vía canónica PI3K/AKT/mTOR y sus alteraciones en cáncer. Horizonte Médico (Lima), 21(4), p. e1547. Available at: https://doi.org/10.24265/horizmed.2021.v21n4.15.
Aquino, M.T.P. et al. (2016). Challenges and future perspectives of T cell immunotherapy in cancer. Immunology Letters, 166(2), pp. 117–133. Available at: https://doi.org/10.1016/j.imlet.2015.05.018.
Avalos, G. et al. (2019). Circulating soluble levels of MIF in women with breast cancer in the molecular subtypes : relationship with Th17 cytokine profile. Clinical and Experimental Medicine, pp. 3–9. Available at: https://doi.org/10.1007/s10238-019-00559-6.
Baker, S.K. and Strickland, S. (2020). A critical role for plasminogen in inflammation. Journal of Experimental Medicine, 217(4). Available at: https://doi.org/10.1084/jem.20191865.
Barzon, V. et al. (2022). Improving the Laboratory Diagnosis of M-like Variants Related to Alpha1-Antitrypsin Deficiency. International Journal of Molecular Sciences, 23(17). Available at: https://doi.org/10.3390/ijms23179859.
Bashir, A. et al. (2016). Novel variants of SERPIN1A gene: Interplay between alpha1-antitrypsin deficiency and chronic obstructive pulmonary disease. Respiratory Medicine, 117, pp. 139–149. Available at: https://doi.org/10.1016/j.rmed.2016.06.005.
Bates, J.P. et al. (2018). Mechanisms of immune evasion in breast cancer. BMC Cancer, 18(1), p. 556. Available at: https://doi.org/10.1186/s12885-018-4441-3.
Belmonte I, Blanco I, Bustamante A, Cadenas S, Casas F, Curí S, Chiner E, Dasí F, Esquinas C, Escribano A, et al. (2016). No TitDéficit de alfa-1 antitripsina: fisiopatología, enfermedades relacionadas, diagnóstico y tratamientole. In Respira. 2da ed, pp. 41–58.
Bergin, D.A. et al. (2014). The Circulating Proteinase Inhibitor α-1 Antitrypsin Regulates Neutrophil Degranulation and Autoimmunity. Science Translational Medicine, 6(217). Available at: https://doi.org/10.1126/scitranslmed.3007116.
Blanchard, V. et al. (2011). N-glycosylation and biological activity of recombinant human alpha1-antitrypsin expressed in a novel human neuronal cell line. Biotechnology and Bioengineering, 108(9), pp. 2118–2128. Available at: https://doi.org/10.1002/bit.23158.
Capoun, O. et al. (2015). Diagnostic Importance of Selected Protein Serum Markers in the Primary Diagnostics of Prostate Cancer. Urologia Internationalis, 95(4), pp. 429–435. Available at: https://doi.org/10.1159/000431364.
Chang, Y.-H. et al. (2012). Secretomic Analysis Identifies Alpha-1 Antitrypsin (A1AT) as a Required Protein in Cancer Cell Migration, Invasion, and Pericellular Fibronectin Assembly for Facilitating Lung Colonization of Lung Adenocarcinoma Cells. Molecular & Cellular Proteomics, 11(11), pp. 1320–1339. Available at: https://doi.org/10.1074/mcp.M112.017384.
Crisford, H., Sapey, E. and Stockley, R.A. (2018). Proteinase 3; a potential target in chronic obstructive pulmonary disease and other chronic inflammatory diseases. Respiratory Research, 19(1), p. 180. Available at: https://doi.org/10.1186/s12931-018-0883-z.
Draxler, D., Sashindranath, M. and Medcalf, R. (2016). Plasmin: A Modulator of Immune Function. Seminars in Thrombosis and Hemostasis, 43(02), pp. 143–153. Available at: https://doi.org/10.1055/s-0036-1586227.
Ehlers, M.R. (2014). Immune-modulating effects of alpha-1 antitrypsin. Biological Chemistry, 395(10), pp. 1187–1193. Available at: https://doi.org/10.1515/hsz-2014-0161.
Enewold, L. et al. (2012). SERPINA1 and ELA2 polymorphisms are not associated with COPD or lung cancer. Anticancer Research, 32(9), pp. 3923–3928.
Ercetin et al. (2019). Clinical Significance of SERPINA1 Gene and Its Encoded Alpha1-antitrypsin Protein in NSCLC. Cancers, 11(9), p. 1306. Available at: https://doi.org/10.3390/cancers11091306.
Foil, K.E. (2021). Variants of SERPINA1 and the increasing complexity of testing for alpha-1 antitrypsin deficiency. Therapeutic Advances in Chronic Disease, 12_suppl, pp. 33–48. Available at: https://doi.org/10.1177/20406223211015954.
Geraghty, P. et al. (2014). α 1 -Antitrypsin Activates Protein Phosphatase 2A to Counter Lung Inflammatory Responses. American Journal of Respiratory and Critical Care Medicine, 190(11), pp. 1229–1242. Available at: https://doi.org/10.1164/rccm.201405-0872OC.
Hanahan, D. (2022). Hallmarks of Cancer: New Dimensions. Cancer Discovery, 12(1), pp. 31–46. Available at: https://doi.org/10.1158/2159-8290.CD-21-1059.
Hanahan, D. and Weinberg, R.A. (2011). Hallmarks of cancer: The next generation. Cell, 144(5), pp. 646–674. Available at: https://doi.org/10.1016/j.cell.2011.02.013.
Hirasawa, Y. et al. (2021). Diagnostic performance of OncuriaTM, a urinalysis test for bladder cancer. Journal of Translational Medicine, 19(1), pp. 1–10. Available at: https://doi.org/10.1186/s12967-021-02796-4.
Jaberie, H., Hosseini, S.V. and Naghibalhossaini, F. (2020). Evaluation of Alpha 1-Antitrypsin for the Early Diagnosis of Colorectal Cancer. Pathology & Oncology Research, 26(2), pp. 1165–1173. Available at: https://doi.org/10.1007/s12253-019-00679-0.
Janciauskiene, S. et al. (2019). Clinical significance of serpina1 gene and its encoded alpha1-antitrypsin protein in nsclc. Cancers, 11(9). Available at: https://doi.org/10.3390/cancers11091306.
Janciauskiene, S. et al. (2021). Potential Roles of Acute Phase Proteins in Cancer: Why Do Cancer Cells Produce or Take Up Exogenous Acute Phase Protein Alpha1-Antitrypsin?. Frontiers in Oncology, pp. 1–10. Available at: https://doi.org/10.3389/fonc.2021.622076.
Janciauskiene, S.M. et al. (2011). The discovery of α1-antitrypsin and its role in health and disease. Respiratory Medicine, 105(8), pp. 1129–1139. Available at: https://doi.org/10.1016/j.rmed.2011.02.002.
Keeratichamroen, S. et al. (2020). Identification of potential cervical cancer serum biomarkers in Thai patients. Oncology Letters, 19(6), pp. 3815–3826. Available at: https://doi.org/10.3892/ol.2020.11519.
Kim, Y. et al. (1999). Nitric Oxide Protects PC12 Cells from Serum Deprivation-Induced Apoptosis by cGMP-Dependent Inhibition of Caspase Signaling’. 19(16), pp. 6740–6747.
Kunder, M., Lakshmaiah, V. and Moideen Kutty, A. V. (2018). Plasma Neutrophil Elastase, α1-Antitrypsin, α2-Macroglobulin and Neutrophil Elastase–α1-Antitrypsin Complex Levels in patients with Dengue Fever. Indian Journal of Clinical Biochemistry, 33(2), pp. 218–221. Available at: https://doi.org/10.1007/s12291-017-0658-1.
Lechowicz, U. et al. (2020). Post-translational modifications of circulating alpha-1-antitrypsin protein. International Journal of Molecular Sciences, 21(23), pp. 1–18. Available at: https://doi.org/10.3390/ijms21239187.
Lim, J.-H. et al. (2020). Alpha-1 antitrypsin inhibits formaldehyde-induced apoptosis of human peritoneal mesothelial cells. Peritoneal Dialysis International: Journal of the International Society for Peritoneal Dialysis, 40(2), pp. 124–131. Available at: https://doi.org/10.1177/0896860819887288.
Ljujic, M. et al. (2016). ALPHA-1 antitrypsin affects U0126-induced cytotoxicity in colon cancer cell line (HCT116). Molecular Biology, 50(1), pp. 153–156. Available at: https://doi.org/10.1134/S002689331601012X.
López-Árias, E. et al. (2012). Alpha 1-antitrypsin: A novel tumor-associated antigen identified in patients with early-stage breast cancer. Electrophoresis, 33(14), pp. 2130–2137. Available at: https://doi.org/10.1002/elps.201100491.
Lorincz, R. and Curiel, D.T. (2020). Advances in alpha-1 antitrypsin gene therapy. American Journal of Respiratory Cell and Molecular Biology, 63(5), pp. 560–570. Available at: https://doi.org/10.1165/RCMB.2020-0159PS.
Manne, V. and Kowdley, K. V. (2020). Alpha1-Antitrypsin Deficiency: A Cause of Chronic Liver Disease. Clinics in Liver Disease, 24(3), pp. 483–492. Available at: https://doi.org/10.1016/j.cld.2020.04.010.
Marando, M., Rayroux, C. and Bergeron, A. (2022). Alpha-1 antitrypsin deficiency. Revue Medicale Suisse, 18(804), pp. 2169–2174. Available at: https://doi.org/10.53738/REVMED.2022.18.804.2169.
Marijanovic, E.M. et al. (2019). Reactive centre loop dynamics and serpin specificity. Scientific Reports, 9(1), pp. 1–15. Available at: https://doi.org/10.1038/s41598-019-40432-w.
Miyake, M. et al. (2013). Investigation of CCL18 and A1AT as potential urinary biomarkers for bladder cancer detection. BMC Urology, 13(1), p. 1. Available at: https://doi.org/10.1186/1471-2490-13-42.
Motawi, T. et al. (2016). Polymorphisms of α1-antitrypsin and Interleukin-6 genes and the progression of hepatic cirrhosis in patients with a hepatitis C virus infection. Balkan Journal of Medical Genetics, 19(2), pp. 35–44. Available at: https://doi.org/10.1515/bjmg-2016-0034.
Ortega, V.E. et al. (2020). The Effects of Rare SERPINA1 Variants on Lung Function and Emphysema in SPIROMICS. American Journal of Respiratory and Critical Care Medicine, 201(5), pp. 540–554. Available at: https://doi.org/10.1164/rccm.201904-0769OC.
Pérez-Holanda, S. et al. (2014). Serum concentration of alpha-1 antitrypsin is significantly higher in colorectal cancer patients than in healthy controls. BMC Cancer, 14(1), p. 355. Available at: https://doi.org/10.1186/1471-2407-14-355.
Remih, K., Amzou, S. and Strnad, P. (2021). Alpha1-antitrypsin deficiency: New therapies on the horizon. Current Opinion in Pharmacology, 59, pp. 149–156. Available at: https://doi.org/10.1016/j.coph.2021.06.001.
De Serres & Blanco (2014). Role of alpha-1 antitrypsin in human health and disease. Journal of Internal Medicine, 276(4), pp. 311–335. Available at: https://doi.org/10.1111/joim.12239.
Shakya, R. et al. (2017). Mutant p53 upregulates alpha-1 antitrypsin expression and promotes invasion in lung cancer. Oncogene, 36(31), pp. 4469–4480. Available at: https://doi.org/10.1038/onc.2017.66.
Stockley, R.A. (2015). α1-antitrypsin: A polyfunctional protein?. The Lancet Respiratory Medicine, 3(5), pp. 341–343. Available at: https://doi.org/10.1016/S2213-2600(15)00094-6.
Strnad, P., McElvaney, N.G. and Lomas, D.A. (2020). Alpha 1 -Antitrypsin Deficiency. New England Journal of Medicine. Edited by D.L. Longo, 382(15), pp. 1443–1455. Available at: https://doi.org/10.1056/NEJMra1910234.
Sun, Z. and Yang, P. (2004). Role of imbalance between neutrophil elastase and α1-antitrypsin in cancer development and progression. Lancet Oncology, 5(3), pp. 182–190. Available at: https://doi.org/10.1016/S1470-2045(04)01414-7.
Tan, S.G., Cunliffe, W.J. and Macgregor, A.J. (1976). Acne Mechanica. British Medical Journal, 1(6002), p. 130. Available at: https://doi.org/10.1136/bmj.1.6002.130.
Thun, G.A. et al. (2013). Causal and Synthetic Associations of Variants in the SERPINA Gene Cluster with Alpha1-antitrypsin Serum Levels. PLoS Genetics. Edited by G. Gibson, 9(8), p. e1003585. Available at: https://doi.org/10.1371/journal.pgen.1003585.
Timms, J.F. et al. (2014). Discovery of serum biomarkers of ovarian cancer using complementary proteomic profiling strategies. Proteomics - Clinical Applications, 8(11–12), pp. 982–993. Available at: https://doi.org/10.1002/prca.201400063.
Tumpara, S. et al. (2020). The Delivery of α1-Antitrypsin Therapy Through Transepidermal Route: Worthwhile to Explore. Frontiers in Pharmacology, 11. Available at: https://doi.org/10.3389/fphar.2020.00983.
Urquidi, V. et al. (2012). Diagnostic Potential of Urinary α1-Antitrypsin and Apolipoprotein E in the Detection of Bladder Cancer. Journal of Urology, 188(6), pp. 2377–2383. Available at: https://doi.org/10.1016/j.juro.2012.07.094.
Verathamjamras, C. et al. (2023). Label-free quantitative proteomics reveals aberrant expression levels of LRG, C9, FN, A1AT and AGP1 in the plasma of patients with colorectal cancer. Clinical Proteomics, 20(1), pp. 1–16. Available at: https://doi.org/10.1186/s12014-023-09407-y.
Vianello, A. et al. (2021). Correlation between α1-antitrypsin deficiency and SARS-CoV-2 infection: Epidemiological data and pathogenetic hypotheses. Journal of Clinical Medicine, 10(19), pp. 1–12. Available at: https://doi.org/10.3390/jcm10194493.
Wang, M. et al. (2017). Mechanism of immune evasion in breast cancer. OncoTargets and Therapy, 10, pp. 1561–1573. Available at: https://doi.org/10.2147/OTT.S126424.
Wu, D.M. et al. (2020). Alpha-1 antitrypsin induces epithelial-to-mesenchymal transition, endothelial-to-mesenchymal transition, and drug resistance in lung cancer cellS. OncoTargets and Therapy, 13, pp. 3751–3763. Available at: https://doi.org/10.2147/OTT.S242579.
Yuan, Y. et al. (2018). Anti-inflammaging effects of human alpha-1 antitrypsin. Aging Cell, 17(1), pp. 1–11. Available at: https://doi.org/10.1111/acel.12694.
Zhao, Z. et al. (2018). Silence of α1-antitrypsin inhibits migration and proliferation of triple negative breast cancer cells. Medical Science Monitor, 24, pp. 6851–6860. Available at: https://doi.org/10.12659/MSM.910665.
Archivos adicionales
Publicado
Cómo citar
Número
Sección
Licencia
Derechos de autor 2023

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-CompartirIgual 4.0.
La revista Biotecnia se encuentra bajo la licencia Atribución-NoComercial-CompartirIgual 4.0 Internacional (CC BY-NC-SA 4.0)















_(2).jpg)








