Aplicación potencial de residuos agroforestales en la adsorción de anilina
DOI:
https://doi.org/10.18633/biotecnia.v26.2416Palabras clave:
aminas aromáticas, residuos, mecanismos de adsorción, carbon activado, isotermasResumen
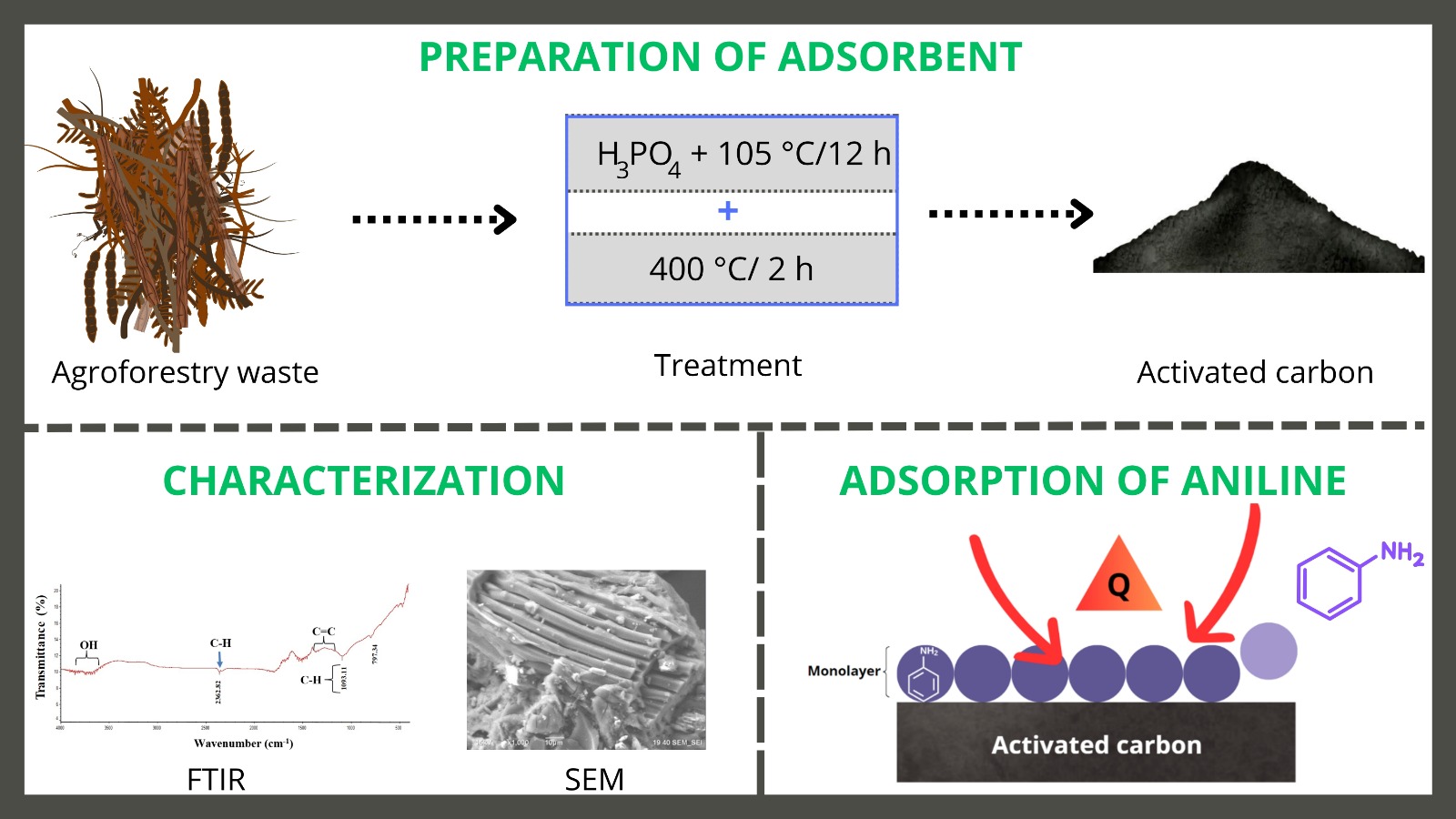
Se sintetizó y caracterizó carbón activado (CAA) de bajo costo a base de residuos agroforestales para la eliminación de anilina (AN), la cual es una sustancia tóxica para la salud y el medio ambiente. Se evaluó el efecto del tiempo de contacto (1, 2, 6, 12 y 24 h), pH (2 ,4 ,6 y 8) con 1, 5 y 10 mg/L de AN. El mecanismo de adsorción de AN en CAA y los carbones comerciales (CAG y CAP) fue evaluado y comparado con las isotermas de Langmuir, Freundlich y Temkin. Los resultados mostraron que la eliminación de AN aumentó con la disminución del pH, el CAA alcanzó una remoción máxima de 90 %. El CAA resultó en una eficiencia similar al CAG > 90 % a 1 y 5 mg/L. El modelo de Langmuir mostró el mejor ajuste con una R2 de 0.98. Estos modelos explican que el mecanismo de adsorción de AN sobre CAA es homogéneo, y se produce adsorción monocapa, alcanzando una capacidad, alcanzando una capacidad máxima de 1.20 y 1.16 mg/g para CAG y CAP respectivamente, con un posible mecanismo endotérmico sugerido por Temkin. El CAA podría considerarse un adsorbente eficaz y económico en la eliminación del AN.
Descargas
Citas
Abin-Bazaine, A., Trujillo, A. C., & Olmos-Marquez, M. 2022. Adsorption isotherms: Enlightenment of the phenomenon of adsorption. Wastewater Treatment, 19, 1-5. Doi:10.5772/intechopen.104260.
Ahmadi S, Kord Mostafapour F. 2017b. Adsorptive removal of bisphenol A from aqueous solutions by Pistacia Atlantica: isotherm and kinetic studies. Pharm Chem J 4:1–8. http://doi.org/10.18801/jstei.050117.35
Ahmadi, S., Mostafapour, F., & Bazrafshan, E. 2017. Removal of aniline and from aqueous solutions by coagulation/flocculation–flotation. Chemical Science International Journal, 18(3), 1-10. https://doi.org/10.9734/CSJI/2017/32016
Asencios, Y. J. O., Parreira, L. M., Perpetuo, E. A., & Rotta, A. L. 2022. Characterization of seaweeds collected from Baixada Santista litoral, and their potential uses as biosorbents of heavy metal cations. Revista Mexicana de Ingeniería Química, 21(1), IA2600-IA2600. https://doi.org/10.24275/rmiq/IA2600
Basiri, H., Nourmoradi, H., Moghadam, F. M., Moghadam, K. F., Mohammadian, J., & Khaniabadi, Y. O. 2015. Removal of aniline as a health-toxic substance from polluted water by aloe vera waste-based activated carbon. Der pharma chemica, 7(11), 149-55.
Benito, A., Penadés, A., Lliberia, J. L., & Gonzalez-Olmos, R. 2017. Degradation pathways of aniline in aqueous solutions during electro-oxidation with BDD electrodes and UV/H2O2 treatment. Chemo-sphere, 166, 230-237. https://doi.org/10.1016/j.chemosphere.2016.09.105
Benjelloun, M., Miyah, Y., Evrendilek, G. A., Zerrouq, F., & Lairini, S. 2021. Recent advances in ad-sorption kinetic models: their application to dye types. Arabian Journal of Chemistry, 14(4), 103031. https://doi.org/10.1016/j.arabjc.2021.103031
Bosch, D., Back, J. O., Gurtner, D., Giberti, S., Hofmann, A., & Bockreis, A. 2022. Alternative feedstock for the production of activated carbon with ZnCl2: Forestry residue biomass and waste wood. Carbon Resources Conversion, 5(4), 299-309. https://doi.org/10.1016/j.crcon.2022.09.001
Brasquet, C., Rousseau, B., Estrade-Szwarckopf, H., & Le Cloirec, P. 2000. Observation of activated carbon fibres with SEM and AFM correlation with adsorption data in aqueous solution. Carbon, 38(3), 407-422. https://doi.org/10.1016/S0008-6223(99)00120-7
Brazesh, B., Mousavi, S. M., Zarei, M., Ghaedi, M., Bahrani, S., & Hashemi, S. A. 2021. Biosorption. In Interface Science and Technology (Vol. 33, pp. 587-628). Elsevier. https://doi.org/10.1016/B978-0-12-818805-7.00003-5
Castellar-Ortega, G., Mendoza C, E., Angulo M, E., Paula P, Z., Rosso B, M., & Jaramillo C, J. 2019. Equilibrium, kinetic and thermodynamic of direct blue 86 dye adsorption on activated carbon obtained from manioc husk. Revista MVZ Córdoba, 24(2), 7231-7238. https://doi.org/10.21897/rmvz.1700
Chaturvedi, N. K., & Katoch, S. S. 2020. Remedial technologies for aniline and aniline derivatives elimination from wastewater. Journal of Health and Pollution, 10(25), 200302. DOI: 10.5696/2156-9614-10.25.200302
Deng, H., Li, Q., Huang, M., Li, A., Zhang, J., Li, Y., ... & Mo, W. 2020. Removal of Zn (II), Mn (II) and Cu (II) by adsorption onto banana stalk biochar: adsorption process and mechanisms. Water Science and Technology, 82(12), 2962-2974. https://doi.org/10.2166/wst.2020.543
Fakhri, A. (2017). Adsorption characteristics of graphene oxide as a solid adsorbent for aniline removal from aqueous solutions: Kinetics, thermodynamics and mechanism studies. Journal of Saudi Chemical Society, 21, S52-S57. https://doi.org/10.1016/j.jscs.2013.10.002
Figueroa, D., Moreno, A., & Hormaza, A. 2015. Equilibrio, termodinámica y modelos cinéticos en la adsorción de Rojo 40 sobre tuza de maíz. Revista Ingenierías Universidad de Medellín, 14(26), 105-120.
Foo, K. Y., & Hameed, B. H. 2010. An overview of dye removal via activated carbon adsorption process. Desalination and Water Treatment, 19(1-3), 255-274. https://doi.org/10.5004/dwt.2010.1214
Ghosh, R. K., Ray, D. P., Tewari, A., & Das, I. 2021. Removal of textile dyes from water by jute stick activated carbon: process optimization and isotherm studies. International Journal of Environmental Science and Technology, 18(9), 2747-2764. https://doi.org/10.1007/s13762-020-03003-5
Guo, L., Li, G., Liu, J., Yin, P., & Li, Q. 2009. Adsorption of aniline on cross-linked starch sulfate from aqueous solution. Industrial & engineering chemistry research, 48(23), 10657-10663. https://doi.org/10.1021/ie9010782
Guo, S., Wang, Z., Wu, S., Cai, Y., Zhang, J., Lou, C., & Zhao, W. 2024. Modification of the adsorption model for the mixture of odor compounds and VOCs on activated Carbon: Insights from pore size dis-tribution. Separation and Purification Technology, 339, 126669. https://doi.org/10.1016/j.seppur.2024.126669
Hameed, B. H., & Rahman, A. A. 2008. Removal of phenol from aqueous solutions by adsorption onto activated carbon prepared from biomass material. Journal of hazardous materials, 160(2-3), 576-581. https://doi.org/10.1016/j.jhazmat.2008.03.028
Hossein, S. K., Hossein, M. M., Reza, A. M., Hassan, H., Mohammad, T., & Maryam, N. 2013. Pre-concentration and Equilibrium Isotherm Studies of Rhodium (III) in Environmental Water Samples. International Journal of Engineering Practical Research, 2(4), 148-155.
Huang, D., Zhang, G., Yi, J., Cheng, M., Lai, C., Xu, P., ... & Chen, S. 2021. Progress and challenges of metal-organic frameworks-based materials for SR-AOPs applications in water treatment. Chemosphere, 263, 127672. https://doi.org/10.1016/j.chemosphere.2020.127672
Iglesias, O., Fernández de Dios, M. A., Pazos, M., & Sanromán, M. A. 2013. Using iron-loaded sepiolite obtained by adsorption as a catalyst in the electro-Fenton oxidation of Reactive Black 5. Environmental Science and Pollution Research, 20, 5983-5993. https://doi.org/10.1007/s11356-013-1610-4
Khaniabadi, Y. O., Heydari, R., Nourmoradi, H., Basiri, H., & Basiri, H. 2016. Low-cost sorbent for the removal of aniline and methyl orange from liquid-phase: aloe vera leaves wastes. Journal of the Taiwan institute of chemical engineers, 68, 90-98. https://doi.org/10.1016/j.jtice.2016.09.025
Khoshnamvand, N., Ahmadi, S., & Mostafapour, F. K. 2017. Kinetic and isotherm studies on ciprof-loxacin an adsorption using magnesium oxide nanopartices. Journal of Applied Pharmaceutical Science, 7(11), 079-083 DOI: 10.7324/JAPS.2017.71112
Kord Mostafapour, F., Ahmadi, Sh., Balarak, D. and Rahdar, S. 2016. Comparison of dissolved air flotation process Function for aniline and penicillin G removal from aqueous solutions. J. Hamadan Univ. Med. Sci. 82(4), 203-209.
Kumar, A., Kumar, S., Kumar, S., & Gupta, D. V. 2007. Adsorption of phenol and 4-nitrophenol on granular activated carbon in basal salt medium: equilibrium and kinetics. Journal of hazardous materials, 147(1-2), 155-166. https://doi.org/10.1016/j.jhazmat.2006.12.062
Liu, Y. B., Qu, D., Wen, Y. J., & Ren, H. J. 2015. Low-temperature biodegradation of aniline by freely suspended and magnetic modified Pseudomonas migulae AN-1. Applied microbiology and biotech-nology, 99, 5317-5326. https://doi.org/10.1007/s00253-015-6399-2
Lu, D., Xu, S., Qiu, W., Sun, Y., Liu, X., Yang, J., & Ma, J. 2020. Adsorption and desorption behaviors of antibiotic ciprofloxacin on functionalized spherical MCM-41 for water treatment. Journal of Cleaner Production, 264, 121644. https://doi.org/10.1016/j.jclepro.2020.121644
Mamaghani, Z. G., Hawboldt, K. A., & MacQuarrie, S. 2023. Adsorption of CO2 using biochar-review of the impact of gas mixtures and water on adsorption. Journal of Environmental Chemical Engineering, 11(3), 109643. https://doi.org/10.1016/j.jece.2023.109643
Muñoz, Y., Arriagada, R., Soto‐Garrido, G., & García, R. 2003. Phosphoric and boric acid activation of pine sawdust. Journal of Chemical Technology & Biotechnology: International Research in Process, Environmental & Clean Technology, 78(12), 1252-1258. https://doi.org/10.1002/jctb.923
Olasehinde, E. F., Abegunde, S. M., & Adebayo, M. A. 2020. Adsorption isotherms, kinetics and thermodynamic studies of methylene blue dye removal using Raphia taedigera seed activated carbon.
Padilla-Ortega, E., Leyva-Ramos, R., & Flores-Cano, J. V. 2013. Binary adsorption of heavy metals from aqueous solution onto natural clays. Chemical Engineering Journal, 225, 535-546. https://doi.org/10.1016/j.cej.2013.04.011
Resasco, D. E., Crossley, S. P., Wang, B., & White, J. L. 2021. Interaction of water with zeolites: a review. Catalysis Reviews, 63(2), 302-362. https://doi.org/10.1080/01614940.2021.1948301
Rosales, E., Ferreira, L., Sanromán, M. Á., Tavares, T., & Pazos, M. 2015. Enhanced selective metal adsorption on optimised agroforestry waste mixtures. Bioresource Technology, 182, 41-49. https://doi.org/10.1016/j.biortech.2015.01.094
Santos, M. P., & Rodrigues, A. E. 2014. Adsorption equilibrium and fixed bed adsorption of aniline onto polymeric resin and activated carbons. Separation Science and Technology, 49(3), 335-344. https://doi.org/10.1080/01496395.2013.852226
Sharma, G., Sharma, S., Kumar, A., Lai, C. W., Naushad, M., Shehnaz, ... & Stadler, F. J. 2022. Acti-vated carbon as superadsorbent and sustainable material for diverse applications. Adsorption Science & Technology, 2022, 4184809. https://doi.org/10.1155/2022/4184809
Shurvell, H. F. 2006. Spectra–structure correlations in the mid‐and far‐infrared. Handbook of vibrational spectroscopy. DOI: 10.1002/9780470027325.s4101
Srivastava, A., Gupta, B., Majumder, A., Gupta, A. K., & Nimbhorkar, S. K. 2021. A comprehensive review on the synthesis, performance, modifications, and regeneration of activated carbon for the ad-sorptive removal of various water pollutants. Journal of Environmental Chemical Engineering, 9(5), 106177. https://doi.org/10.1016/j.jece.2021.106177
Sun, Y., Wang, T., Sun, X., Bai, L., Han, C., & Zhang, P. 2021. The potential of biochar and lignin-based adsorbents for wastewater treatment: Comparison, mechanism, and application—A review. Industrial Crops and Products, 166, 113473. https://doi.org/10.1016/j.indcrop.2021.113473
Tay, T., Ucar, S., & Karagöz, S. 2009. Preparation and characterization of activated carbon from waste biomass. Journal of hazardous materials, 165(1-3), 481-485. https://doi.org/10.1016/j.jhazmat.2008.10.011
Temkin, M.I., 1940. Kinetics of ammonia synthesis on promoted iron catalysts. Acta Physiochimica URSS 12, 327–356.
Tetteh, I. K., Issahaku, I., & Tetteh, A. Y. 2024. Recent advances in synthesis, characterization, and environmental applications of activated carbons and other carbon derivatives. Carbon Trends, 100328. https://doi.org/10.1016/j.cartre.2024.100328
Varghese, S. M., Chowdhury, A. R., Arnepalli, D. N., & Rao, G. R. 2024. Crosslinked hydrogel-derived carbons activated by trace amounts of aqueous potassium carbonate for carbon dioxide adsorption. Bioresource Technology, 403, 130851. https://doi.org/10.1016/j.biortech.2024.130851
Vázquez-Sánchez, A. Y., Lima, E. C., Abatal, M., Tariq, R., Santiago, A. A., Alfonso, I., ... & Vazquez-Olmos, A. R. 2023. Biosorption of Pb (II) using natural and treated Ardisia compressa K. leaves: Simulation framework extended through the application of artificial neural network and genetic algorithm. Molecules, 28(17), 6387. https://doi.org/10.3390/molecules28176387
Vijan, L. E., & Neagu, M. 2012. Adsorption isotherms of phenol and aniline on activated carbon. Revue Roumaine de Chimie, 57(2), 85-93.
Weber, T. W., & Chakravorti, R. K. 1974. Pore and solid diffusion models for fixed‐bed adsorbers. AIChE Journal, 20(2), 228-238. https://doi.org/10.1002/aic.690200204
Wu, C. D., Zhang, J. Y., Wang, L., & He, M. H. 2013. Removal of aniline and phenol from water using raw and aluminum hydroxide-modified diatomite. Water science and technology, 67(7), 1620-1626. https://doi.org/10.2166/wst.2013.038
Yakout, S. M., & Ali, M. S. 2015. Removal of the hazardous crystal violet dye by adsorption on corn-cob-based and phosphoric acid-activated carbon. Particulate Science and Technology, 33(6), 621-625. https://doi.org/10.1080/02726351.2015.1016642
Yan, Z., Gu, Y., Wang, X., Hu, Y., & Li, X. 2021. Degradation of aniline by ferrous ions activated persulfate: impacts, mechanisms, and by-products. Chemosphere, 268, 129237. https://doi.org/10.1016/j.chemosphere.2020.129237
Yunus, Z. M., Al-Gheethi, A., Othman, N., Hamdan, R., & Ruslan, N. N. 2022. Advanced methods for activated carbon from agriculture wastes; a comprehensive review. International Journal of Environ-mental Analytical Chemistry, 102(1), 134-158. https://doi.org/10.1080/03067319.2020.1717477
Zhou, Y., Lu, J., Zhou, Y., & Liu, Y. 2019. Recent advances for dyes removal using novel adsorbents: a review. Environmental pollution, 252, 352-365. https://doi.org/10.1016/j.envpol.2019.05.072
Zhu, R., Yu, Q., Li, M., Zhao, H., Jin, S., Huang, Y., & Chen, J. 2021. Analysis of factors influencing pore structure development of agricultural and forestry waste-derived activated carbon for adsorption application in gas and liquid phases: A review. Journal of Environmental Chemical Engineering, 9(5), 105905. https://doi.org/10.1016/j.jece.2021.105905
Descargas
Archivos adicionales
Publicado
Cómo citar
Número
Sección
Licencia
Derechos de autor 2023

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-CompartirIgual 4.0.
La revista Biotecnia se encuentra bajo la licencia Atribución-NoComercial-CompartirIgual 4.0 Internacional (CC BY-NC-SA 4.0)















_(2).jpg)








