Full factorial designs of experiments to optimize an ELISA that detects SARS-CoV-2 anti-RBD antibodies in breast milk
ELISA that detects SARS-CoV-2 anti-RBD antibodies in breast milk
DOI:
https://doi.org/10.18633/biotecnia.v25i3.2012Keywords:
Abdala vaccine; effect size; experimental design; partial eta-squareAbstract
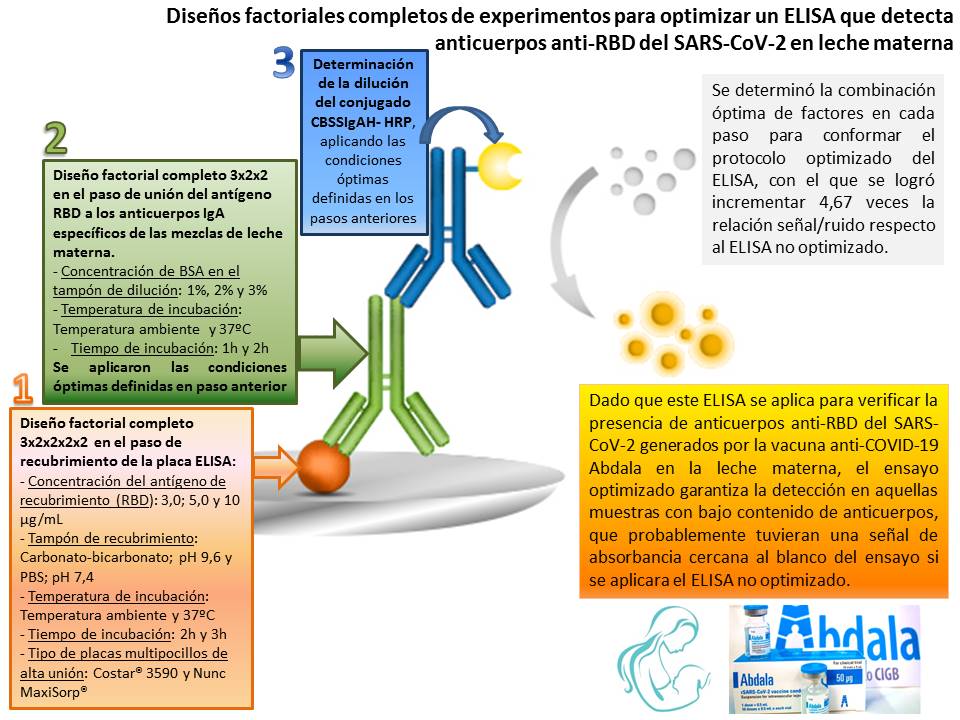
The full factorial design of experiments has been used infrequently to optimize immunoassays, despite its advantages in saving time and resources and its reliable results. The objective of this work was to optimize an ELISA, which detects SARS-CoV-2 RBD-specific IgA antibodies, by applying complete factorial experiments in various steps of the assay. The interactions between all factors and levels at each step were evaluated, using mixtures of breast milk, with high and low antibody content, collected from puerperal women vaccinated with the Abdala anti-COVID-19 vaccine. The optimal combination of factors and levels was determined to form the optimized ELISA protocol, with which it was possible to increase the signal to noise ratio by 4.67 times with respect to the non-optimized protocol. Since this ELISA is applied to verify the presence of SARS-CoV-2 RBD-specific IgA antibodies generated by Abdala vaccine in breast milk, the optimized assay guarantees detection in those samples with low antibody content, which probably had an absorbance signal close to the assay blank if the initial non-optimized protocol were applied.
Downloads
References
Ahmed, R.K., Saad, E.M., Fahmy, H.M. y El Nashar, R. M. 2022. Multivariate experimental design: to-wards more reliable electrochemical detection. Current Opinion in Electrochemistry. 31: 100880.
Altekar, M., Homon, C.A., Kashem, M.A., Mason, S.W., Nelson, R.M., Patnaude, L.A., Yingling, J. y Taylor, P. B. 2007. Assay optimization: a statistical design of experiments approach. Clinics in laboratory medicine. 27(1): 139-154.
Augustine, S.A., Simmons, K.J., Eason, T.N., Griffin, S.M., Curioso, C.L., Wymer, L.J., Shay Fout, G., Grimm, A.C., Oshima, K.H. y Dufour, A. 2015. Statistical approaches to developing a multiplex immunoassay for determining human exposure to environmental pathogens. Journal of Immuno-logical Methods. 425: 1-9.
Ben-Shachar, M.S., Lüdecke, D. y Makowski, D. 2020. Effect size: Estimation of effect size indices and standardized parameters. Journal of Open Source Software. 5(56): 2815.
Cohen, J. 1988. The concepts of power analysis. Statistical power analysis for the behavioral sciences. 2: 1-17.
Crowther, J.R. y Smith, H. 1992. ELISA Manual U.K. p.2-21.
Kechagias, J.D., Aslani, K.E., Fountas, N.A., Vaxevanidis, N.M. y Manolakos, D.E. 2020. A comparative investigation of Taguchi and full factorial design for machinability prediction in turning of a titanium alloy. Measurement. 151: 107213.
Minic, R. y Zivkovic, I. 2020. Optimization, validation and standardization of ELISA. Norovirus. 9-28.
Peng, P., Liu, C., Li, Z., Xue, Z., Mao, P., Hu, J., Xu, F., Yao, C. y You, M. (2022). Emerging ELISA derived technologies for in vitro diagnostics. TrAC Trends in Analytical Chemistry. 116605.
Ray, C.A., Patel, V., Shih, J., Macaraeg, C., Wu, Y., Thway, M., Lee, J.W. y DeSilva, B. 2009. Application of multi-factorial design of experiments to successfully optimize immunoassays for robust measurements of therapeutic proteins. Journal of Pharmaceutical and Biomedical Analysis. 49(2): 311-318.
Downloads
Additional Files
Published
How to Cite
Issue
Section
License
Copyright (c) 2023

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
The journal Biotecnia is licensed under the Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0) license.




_(1)_(1).png)






_(2).jpg)





