Nematodes of cucumber (Cucumis sativus) and effect of growth-promoting rhizobacteria on Meloidogyne incognita (Tylenchida: Heteroderidae)
DOI:
https://doi.org/10.18633/biotecnia.v26.2143Keywords:
Bacillus vallismortis, Bacillus velezensis, Biological control, Root knot nematode, Pseudomonas fluorescensAbstract
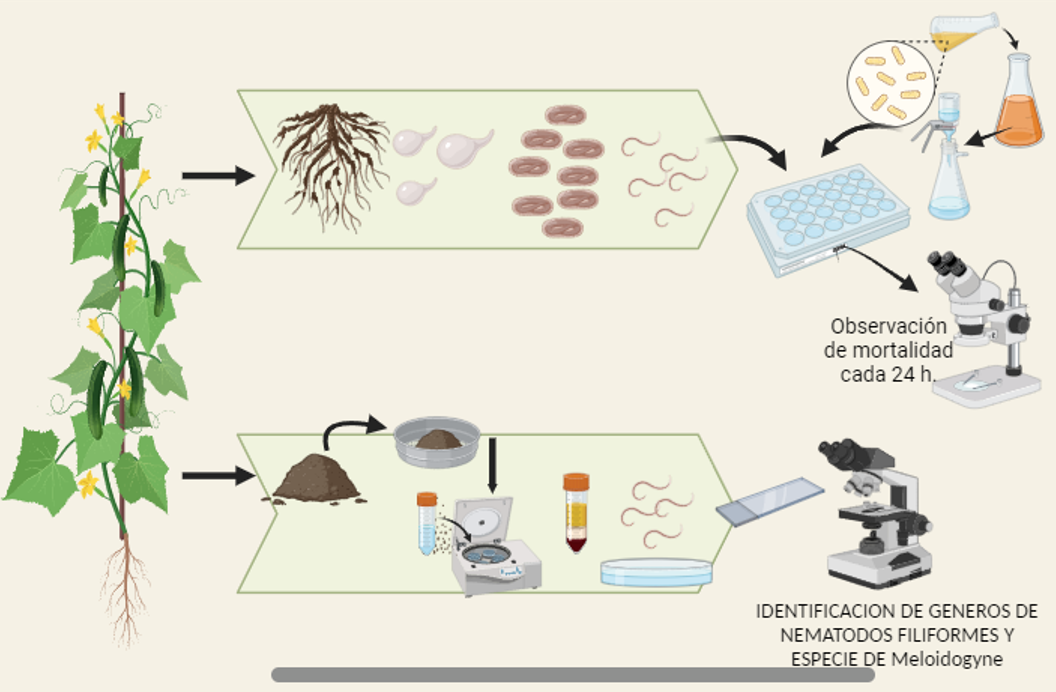
Soil and root samples of the cucumber crop were analyzed. Plant pathogenic nematodes present were extracted and identified. The juveniles of the root-knot nematode Meloidogyne incognita were the most abundant in soil, other such as Aphelenchoides sp., Pratylenchus sp., Tylenchorynchus sp., and Aphelenchus sp. were found in less presence. Females and eggs of M. incognita were obtained from the roots. Finding alternatives to chemicals fumigants, the rhizobacterias Bacillus vallismortis, Bacillus velezensis and Pseudomonas fluorescens were fermented and filtered to be evaluated on these filiform nematodes under laboratory conditions, observing their effect every 24 h. The treatments were evaluated in concentrations of 100% and 50%. The P. fluorescens filtrate was the treatment with the best nematicidal activity, causing a mortality of 95 % in the highest concentration and 93 % in the 50 % concentration at 24 h, followed by the treatments of B. vallismortis and B. velezensis with 83 and 77 % respectively in concentrations of 100 % product. The three treatments showed 100 % mortality of the nematodes after 48 hours of exposure This indicates that these rhizobacteria contain metabolites with nematicidal action that could be a healthy alternative for the control of these phytopathogens.
Downloads
References
Araya, M., Centeno, M., y Carrillo, W. 1995. Densidad poblacional y frecuencia de los nematodos parásitos de banano (Musa AAA) en nueve cantones de Costa Rica. Costa Rica. Corbana 20(43):6 – 11.
Baldwin, J. G., Nadler, S. A., Adams, B. J. 2004. Evolution of plant parasitism among nematodes. Annu Rev Phytopathol. 42:83-105. doi: 10.1146/annurev.phyto.42.012204.130804. PMID: 15283661.
Cohn, F. 1872. Untersuchungen über Bakterien. Pages 127-224 in: Beiträge zur Biologie der Pflanzen 1, 1875 (Heft 1), J. U. Kern’s Verlag (Max Müller), Breslau. Recuperado de https://www.biodiversitylibrary.org/page/5105059#page/2/mode/1up.
Collange, B., Navarrete, M., Peyre, G., Mateille, T., Tchamitchian. 2011. Rootknot nematode (Meloidogyne) management in vegetable crop production: the challenge of an agronomic system analysis. Crop Protection 30(10), 1251–1262.
Condemarin, Carlos. 2018. Efecto de bacterias nativas del suelo cultivado y prístino sobre el control del nematodo agallador radicular, Meloidogyne javanica. en condiciones in vitro y producción de bioma-sa. Arnaldoa, 25(2), 515-528.doi:10.22497/arnaldoa.252.25211.
Cronin, D., Moënne-Loccoz and Dunne, C. 1997. Inhibition of egg hatch of the potato cyst nema-tode Globodera rostochiensis by chitinase-producing bacteria. European Journal of Plant Pathology,103, 433–440. doi:10.1023/A:1008662729757
Dowling D. N. and O'Gara F. 1994. Metabolites of Pseudomonas involved in the biocontrol of plant disease.Trends Biotechnol.12(4)133-144. doi:10.1016/0167-7799(94)90091-4
Eisenback, D.J., Hirschmann, H., Sasser, N.J. y Triantaphyllou, C.A. 1983. Guía para la identificación de las cuatro especies más comunes del nematodo agallador (Meloidogyne especies), con una clave pictórica. International Meloidogyne Project. Raleigh, North Carolina, USA.
EPPO. 2017. PM 3/83 (1) Fragaria plants for planting inspection of places of production. Bulletin OEPP/ EPPO. Bulletin. 43(3),471-495. doi: 10.1111/epp.12408.
Frankland, G. C. and Frankland, P. F. 1887. Studies on some new micro-organisms obtained from air. Philosophical Transactions of the Royal Society of London. B, 178, 257–287.
Flügge C. 1886. Die Mikroorganismen. 2. Aufl. 692 Verlag von F. C. W. Vogel, Leipzig
Gupta, R. C., Miller Mukherjee I. R., Doss R. B., Malik J. K. and Milatovic D. 2017. Organophos-phates and Carbamates. Reproductive and Developmental Toxicology (2), Academic Press, 609-631.
Haas and Defago, G. 2005. Biological control of soil-borne pathogens by fluorescent pseudo-monads. Nat Rev Microbiol,3(4),307–319. doi:10.1038/nrmicro1129.
Hajihassani, A. Davis, R. Timper, P. 2019. Evaluation of Selected Nonfumigant Nematicides on Increasing Inoculation Densities of Meloidogyne incognita on Cucumber. Plant Dis. (12) 3161-3165. doi: 10.1094/PDIS-04-19-0836-RE.
Handoo, Z.A. Khan, A. Islam, S. 2007. A key and diagnostic compendium to the species of the ge-nus Merlinius Siddiqi, 1970 (Nematoda:Tylenchida) with description of Merlinius khuzdarensis n.sp. associated with datepalm Nematology, 9(2),251–260.
Idowu, J. Rotifa, Kenneth A. Evans. 2021. Use of acibenzolar-S-methyl and other novel products for the management of Aphelenchoides fragariae on ornamental plants in glasshouse and commercial conditions. Crop Protection, 141. doi: 10.1016/j.cropro.2020.105467
Jones J. G., Kleczewski N. M., Desaeger J., Meyer S. F. L. 2016. Evaluation of nematicides for southern root-knot nematode management in lima bean. Crop Protection, 96,151-157.
Kaur, P.K., Joshi, N., Singh, I.P., and Saini, H.S. 2017. Identification of cyclic lipopeptides pro-duced by Bacillus vallismortis R2 and their antifungal activity against Alternaria alternata. J Appl Microbiol,122(1),139–152. doi: 10.1111/jam.13303.
Keel, C., Schnider, U., Maurhofer, M., Voisard, C., Laville, J., Burger, U., Wirthner, P., Haas, D., De´fago, G. 1992. Suppression of root diseases by Pseudomonas fluorescens CHA0: importance of the bacterial secondary metabolite 2,4-diacetylphloroglucinol. Molecular Plant– Microbe Interac-tion 5(1), 4–13.
Kohl, L. 2011. Foliar Nematodes: A Summary of Biology and Control with a Compilation of Host Range. Plant Health Progress 12(1), 23. doi:10.1094/php-2011-1129-01-rv
Liu, X. Y., Wang, B. J., Jiang, C. Y., and Liu, S. J. 2007. Micrococcus flavus sp. nov., isolated from activated sludge in a bioreactor. International journal of systematic and evolutionary Microbiolo-gy, 57(1), 66-69. doi:10.1099/ijs.0.64489-0
Liu Y., Teng, K., Wang, T., Dong, E., Zhang, M., Tao, Y., Zhong, J. 2020. Antimicrobial Bacillus velezensis HC6: production of three kinds of lipopeptides and biocontrol potential in maize. J Appl Microbi-ol.128(1):242-254. doi: 10.1111/jam.14459.
Machado, A.C.Z., Dorigo, O.F., Mattei, D. 2013. First Report of the Root Knot Nematode, Meloidogyne inornata, on Common Bean in Paraná State, Brazil. Plant Disease, 97(3), 431-431.
Mai, W. F.; Mullin, P. G.; Lyon, H.H.; and Loeffler, K. 1996. Plant-parasitic nematodes. A Pictorial Key to Genera. Cornell University Press. Nueva York. 277.
Maung, E.C.H., Choi, T.G., Nam, H.H., Kim, K.Y. 2017. Role of Bacillus amyloliquefaciens Y1 in the Control of Fusarium Wilt Disease and Growth Promotion of Tomato. Biocontrol Sci. Technol. 27, 1400–1415 doi:10.1080/09583157.2017.1406064
McNear Jr., D. H. 2013. The rhizosphere - roots, soil and everything in between. Nat. Educat. Knowledge 4 (3):1.
Migula W. 1895. "Bacteriaceae (Stabchenbacterien)." In: Engler and Prantl (Editors), Die Naturlichen Pflanzenfamilien. W. Engelmann, Leipzig, 20-30.
Montes–Belmont, R. 2002. Nematología vegetal en México. Sociedad Mexicana de Fitopatología. Sonora, México. 98 p
Ongena, M., Jacques, P. 2008. Bacillus lipopeptides: Versatile weapons for plant disease biocon-trol. Trends Microbiol,16, 115–125. doi: 10.1016/j.tim.2007.12.009. PMID: 18289856.
Ornat, C., Verdejo-Lucas, S., Sorribas. F.J. 1997. Effect of the previous crop on population densi-ties of Meloidogyne javanica and yield of cucumber. Nematropica (27), 85– 90.
Podile, A. R., Kishore, G. K. 2007. Plant growth-promoting rhizobacteria. In S.S. Gnanamanickam (Ed.) Plant-associated bacteria. Springer, Dordrecht,195–230. doi: 10.1007/978-1-4020-4538-76.
Prasad, S., Tilak, K., and Gollakota, R. 1972. Role of Bacillus thuringiensis var. thuringiensis on the larval survivability and egg hatching of Meloidogyne. spp. The causative agent of root-knot disease. Journal of Invertebrate Pathology, 20. 377-378
Priest, F. G., Goodfellow, M., Shute, L. A., & Berkeley, R. C. W. (1987). Bacillus amyloliquefa-ciens sp. nov., nom. rev. International journal of systematic and evolutionary microbiology, 37(1), 69-71. doi:10.1099/00207713-37-1-69.
Radwan, M., Farrag, S., Abu-Elamayem, M., Ahmed, N. 2012. Biological control of the root-knot nematode, Meloidogyne incognita on tomato using bioproducts of microbial origin. Applied Soil Ecology. 56, 58-62, doi: 10.1016/j.apsoil.2012.02.008.
Ravichandra N. G. 2014. Horticultural Nematology. Springer India, 411. doi: 10.1007/978-81-322-1841-8.
Roberts, M., Nakamura, L., Cohan, F. 1996. Bacillus vallismortis sp. nov., a close relative of Bacil-lus subtilis isolated from soil in Death Valley, California. Int J Syst Bacteriol. 46 (2),470–5.doi:10.1099/00207713-46-2-470.
Ruiz-García, C., Bejar, V., Martinez-Checa, F., Llamas, I., Quesada, E., 2005; Bacillus velezensis sp. nov., a surfactant-producing bacterium isolated from the river Velez in Malaga, southern Spain. Int J Syst Evol Microbiol. 55:191–5. doi:10.1099/ijs.0.63310-0.
Sánchez-Monge, A., Flores, L., Salazar, L., Hockland, S.H., Bert, W. 2015. An updated list of the plants associated with plant-parasitic Aphelenchoides (Nematoda: Aphelenchoididae) and its implications for plant-parasitism within this genus. Zootaxa, 4013 (2), 207-24. doi: 10.11646/zootaxa.4013.2.3
Santos, M., Nogueira, M., Hungria, M. 2019. Microbial inoculants: reviewing the past, discussing the present and previewing an outstanding future for the use of beneficial bacteria in agriculture. AMB Express 9,205. doi: 10.1186/s13568-019-0932-0.
Servicio de Información Agroalimentaria y Pesquera. SIAP. (2023). Anuario Estadístico de la Producción Agrícola. Recuperado de https://nube.siap.gob.mx/cierreagricola/
Siddiqi, R. 2000. Tylenchida: Parasites of plants and insects. CAB International. Londres. 848 p.
Siddiqui, I. A., and S. S. Shaukat. 2003. Plant species, host age and host genotype effects on Meloidogyne incognita biocontrol by Pseudomonas fluorescens strain CHA0 and its genetical-ly-modified derivatives. Journal of Phytopathology,151(4),231-238.
Théatre, A., Cano-Prieto, C., Bartolini, M., Laurin, Y., Deleu, M., Niehren, J.; Fida, T., Gerbinet, S., Alanjary, M., Medema, M.H. 2021. The surfactin-like lipopeptides from Bacillus spp.: Natural biodiver-sity and synthetic biology for a broader application range. Front. Bioeng. Biotech-nol,2(9),623-701. doi: 10.3389/fbioe.2021.623701.
Tian, X., Zhao, X., Zhao, S., Zhao, J., Mao, Z. 2022. The Biocontrol Functions of Bacillus velezen-sis Strain Bv-25 Against Meloidogyne incognita. Front Microbiol ,7(13),843. doi: 10.3389/fmicb.2022.843041.
Villain, L., Anzueto, F., Hernández, A., Sarah, J. 1999. Los nematodos parásitos del cafeto. En: Desafios de la caficultura en Centroamérica. (pp. 327-367). Francia. 496 p Editorial: B. Bertrand y B. Rapidel. CIRAD.
Wang G. L., Chen X. L., Chang Y. N., Du D., Li Z. and Xu X. Y. 2015. Synthesis of 1,2,3-benzotriazin-4-one derivatives containing spirocyclic indoline-2-one moieties and their nematicidal evaluation. Chinese Chemical Letters, 26(12), 1502-1506. doi: 10.1016/j.cclet.2015.10.024
Yeates, G. 1999. Effects of plants on nematode community structure. Ann Rev Phytopathol. 37(1):127-149. doi:10.1146/annurev.phyto.37.1.127.
Zasada, I. A., Walters, T. W. y Pinkerton, J. N. 2010. Post-plant nematicides for the control of root-lesion nematode in red raspberry. HortTechnology 20:856–862.
Zhao, Z., Wang, Q., Wang, K., Brian, K., Liu, C., Gu, Y. 2010. Study of the antifungal activity of Bacillus vallismortis ZZ185 in vitro and identification of its antifungal components. Bioresour Tech-nol.101(1):292-7. doi: 10.1016/j.biortech.2009.07.071. Epub 2009 Aug 29. PMID: 19717300.
Downloads
Additional Files
Published
How to Cite
Issue
Section
License
Copyright (c) 2023

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
The journal Biotecnia is licensed under the Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0) license.
















_(2).jpg)







