Biosurfactant activity of two chitinolytic Bacillus subtilis strains and their antagonism against Corynespora cassiicola
DOI:
https://doi.org/10.18633/biotecnia.v27.2362Keywords:
Biocontrol, inhibition, detached leaves, surfactin, chitinaseAbstract
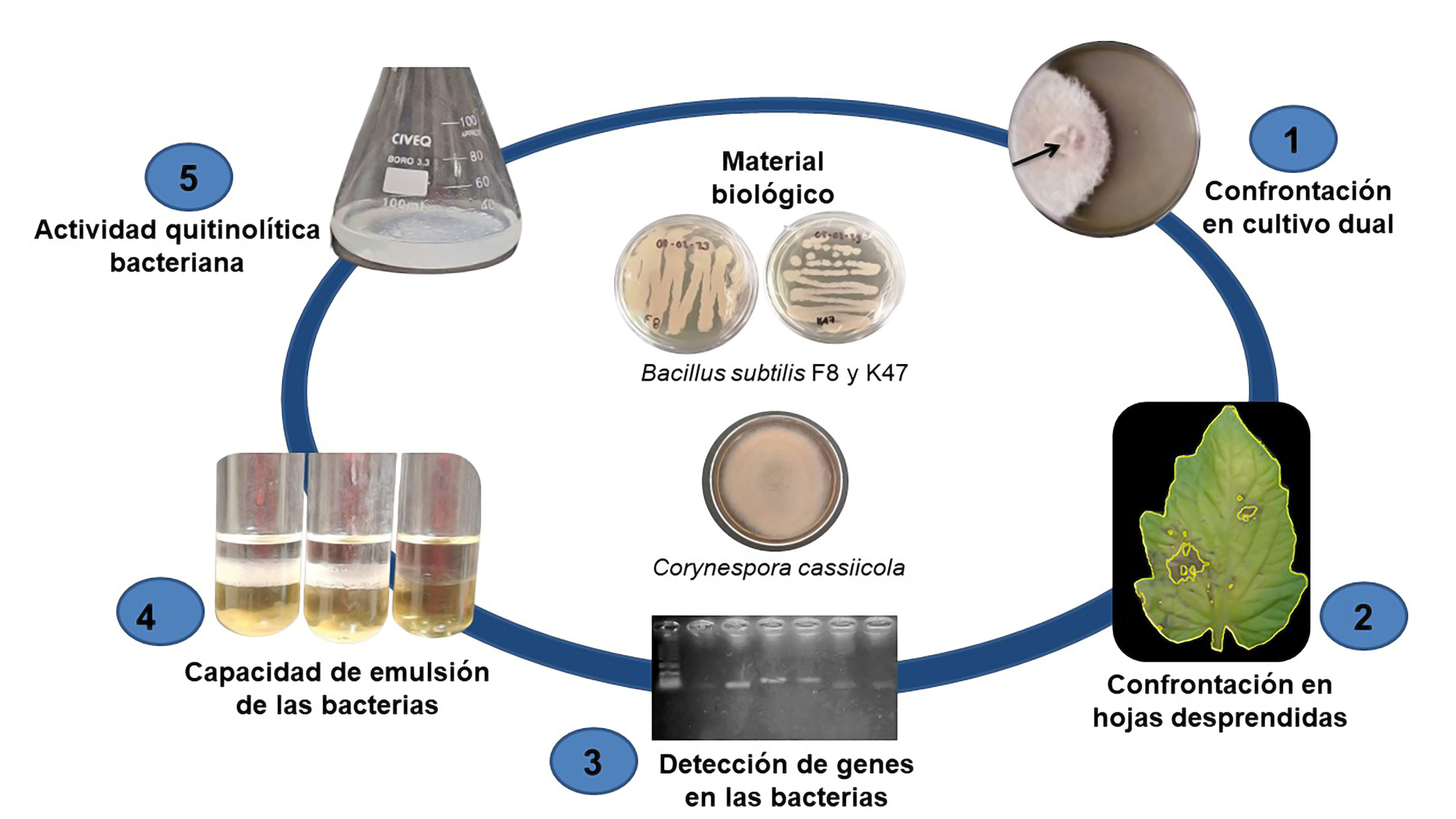
Corynespora cassiicola causes losses in tomato production, agrochemicals are used to control it. Rhizobacteria, such as Bacillus spp. they represent an alternative to chemical products. The effect of Bacillus subtilis F8 and K47 against C. cassiicola was evaluated, mycelial and fungal growth was determined and the percentage of inhibition was calculated. Damage by the pathogen was evaluated in detached leaves inoculated with spores or cell-free filtrate. In B. subtilis, the presence of genes for surfactin biosynthesis, its emulsifying capacity and chitinolytic activity were determined. B. subtilis F8 and K47 reduced the mycelial growth of C. cassiicola 5.6 cm and 5.5 cm and the fungal growth by 79.4 % and 75.6 %, with an inhibition percentage of 69.1 % and 72.9 % respectively. On detached leaves, the application of spores or filtrates reduced disease symptoms by up to 63.8 %. Genes related to surfactin biosynthesis were detected in both strains, with an emulsifying capacity of 21.15 and 21.48 % and a chitinolytic activity of 0.165 μM mg-1 and 0.367 μM mg-1 respectively. B subtilis K47 and F8 present promising potential as an alternative in the control of C. cassiicola in tomato crops.
Downloads
References
Abbott, W.S. 1925. A method of computing the effectiveness of an insecticide. Journal of Economic Entomology. 18: 265-267. http://dx.doi.org/10.1093/jee/18.2.265a.
Al-Mutar, D.M.K., Noman, M., Alzawar, N.S.A., Qasim. H.H., Li, D. and Song, F. 2023. The Extracellular Lipopeptides and Volatile Organic Compounds of Bacillus subtilis DHA41 Display Broad-Spectrum Antifungal Activity against Soil-Borne Phytopathogenic Fungi. Journal Fungi (Basilea). 28: 797. https://doi: 10.3390/jof9080797.
Arun, K.S., Ramachandra, R., Sachin, T., Vishnupriya, G., Piyush, V., Ritu, R. and Keyur, R. 2024. En-gineering a recombinant chitinase from the marine bacterium Bacillus aryabhattai with targeted ac-tivity on insoluble crystalline chitin for chitin oligomer production. International Journal of Bio-logical Macromolecules. 264: 130499. https://doi.org/10.1016/j.ijbiomac.2024.130499.
Athukorala, S. Dilantha, W.G. and Rashid, K.Y 2009. Identification of antifungal antibiotics of Bacillus species isolated from different microhabitats using polymerase chain reaction and MALDI-TOF mass spectrometry. Canadian Journal of Microbiology. 55: 1021-1031.
Aydi Ben Abdallah, R., Jabnoun-Khiareddine, H., Nefzi, A., Mokni-Tlili, S. and Daami-Remadi, M. 2015. Endophytic Bacteria from Datura stramonium for Fusarium wilt suppression and tomato growth promotion. Journal of Microbial & Biochemical Technology. 8: 30e41. DOI: 10.4172/1948-5948.1000259
Aydi Ben Abdallah, R., Stedel, C., Garagounis, C., Nefzi, A., Jabnoun-Khiareddine, H., Papadopoulou, K.K. and Daami-Remadi, M. 2017. Involvement of lipopeptide antibiotics and chitinase genes and induction of host defense in suppression of Fusarium wilt by endophytic Bacillus spp. in tomato. Crop Protection. 99: 45-58. https://doi.org/10.1016/j.cropro.2017.05.008.
Bañuelos, J.J. 2007. Evaluación destructiva de la patogenecidad de Macrophomina phaseolina (Tassi) Goid. en frijol (Phaseolus vulgaris L.) Revista Mexicana de Fitopatología. 26: 71-75. https://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S0185-33092008000100011.
Barboza, J.E., Nieto E, Velazquez, R., Salcedo, R., Bautista, M. and Jimenez, B., Ibarra, J. 2003. Cloning, Sequencing, and Expression of the Chitinase Gene chiA74 from Bacillus thuringiensis. Applied and environmental microbiology. 69: 1023-1029. https://DOI: 10.1128/AEM.69.2.1023–1029.2003.
Becerra, L.K. y Horna, M.V. 2016. Aislamiento de microorganismos productores de biosurfactantes y lipasas a partir de efluentes residuales de camales y suelos contaminados con hidrocarburos. Scientia Agropecuaria. 7: 23-31. http://revistas.unitru.edu.pe/index.php/scientiaagrop.
Bodour, A. A., Guerrero, C., Jiorle, B.V., Paull, A.K., Somogyi, A., Trinh, L.N., Bates, R.B. y Maier, R.M. 2004. Structure and characterization of favolipids, a novel class of biosurfactants produced by Flavobacterium sp. Strain MTN11. Applied and Environmental Microbiology. 70: 114–120. https://doi: 10.1128/AEM.70.1.114-120.2004.
Brzezinska, M.S., Kalwasińska, A., Świątczak, J., Żero, K. and Jankiewicz, U. 2020. Exploring the prop-erties of chitinolytic Bacillus isolates for the pathogens biological control. Microbial Pathogenesis. 148: 104462. https://doi.org/10.1016/j.micpath.2020.104462.
Chávez, G.M. and Cruz, R. 1984. El sistema quitinolítico de Serratia marcescens. Revista Latinoamericana de Microbiología. 26: 203-215.
De la Caridad, A.O., González, R., Díaz, F.R., Reyes, C., Gil, Y., Reyes, S. y Barroso, J. 2017. Aplicación del software ImageJ® 1.43u en la caracterización de los síntomas de la mancha anular de la caña de azúcar. Centro Agrícola. 44: 83-88. http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S0253-57852017000200011&lng=es&tlng=es.
Dong, L., Wang, P., Zhao, W., Su, Z., Zhang, X., Lu, X., Li, S., Ma, P. and Guo, Q. 2022. Surfactin and fengycin contribute differentially to the biological activity of Bacillus subtilis NCD-2 against Cotton Verticillium wilt. Biological Control. 174: https://doi.org/10.1016/j.biocontrol.2022.104999.
Gao, S., R. Zeng, L. Xu, Z. Song, P. Gao. and F. Dai. 2020. Genome sequence and spore germination-associated transcriptome analysis of Corynespora cassiicola from cucumber. BMC Microbiology. 20:199. https://doi: 10.1186/s12866-020-01873-w.
Ghosh, S.K. and Panja, A.. 2021. Different mechanisms of signaling pathways for plant protection from diseases by fungi. In: Biocontrol Agents and Secondary Metabolites. Sudisha Jogaiah. Woodhead Publishing (ed). pp 591-630. ISBN 9780128229194. https://doi.org/10.1016/B978-0-12-822919-4.00026-0.
Kalai-Grami, L., Saidi, S., Bachkouel, S., Ben Slimene, I., Mnari-Hattab, M., Hajlaoui, M.R. and Limam, F. 2014. Isolation and characterization of endophytic bacteria antagonistic to Phoma tracheiphila and Verticillium alboatrum. Applied Biochemistry and Biotechnology. 174: 365e375.
Kumar, K., Pal, G., Verma, A., Kumar, D., Shukla, P., Verma, S. 2023. Seed vectored bacterial endophyte Bacillus pumilus protect sorghum (Sorghum bicolor L.) seedlings from a fungal pathogen Rhizoctonia solani. Biological Control. 183: 105249. https://doi.org/10.1016/j.biocontrol.2023.105249.
Le quoc, D. U. Y. 2023. Evaluation of antifungal activity against Corynespora cassiicola by bacteria iso-lated from soil in the root zone of cucumber plants. Biodiversitas Journal of Biological Diversity. 24: 6584-6591. https://doi.org/10.13057/biodiv/d241220.
Leal, F., Voltolini, R., Velho, D., Gomes, G., Ritter, A., Mui, S. and Brandelli, A. 2015. Expression of essential genes for biosynthesis of antimicrobial peptides of Bacillus is modulated by inactivated cells of target microorganisms. Research in Microbiology. 1-7. http://dx.doi.org/10.1016/j.resmic.2015.10.005.
Mejía, M.A., Cristóbal, J., Tun, J.M. y Reyes, A. 2016. Actividad in vitro de Bacillus spp. en la inhibición de crecimiento micelial de Fusarium equiseti y Fusarium solani aislado de chile habanero (Capsicum chinense Jacq.). Agrociencia, 50: 1123-1135. http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S14051952016000801123&lng=es&tlng=es.
Miller, G.L. 1959. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry. 31: 426-428.
Monreal, J. and Reese, E.T. 1969. The chitinase of Serretia marcescens. Canadian Journal Microbiology. 15: 689-696.
Moo, F.A., Cristóbal, J., Tun, J.M., Medina, I.L., Arjona, A.A. Gamboa M.M. 2022. Activity of aqueous extracts from native plants of the Yucatan Peninsula against fungal pathogens of tomato in vitro and from croton chichenensis against Corynespora cassiicola on tomato. Plants. 11: 2821. https://doi.org/10.3390/plants11212821.
Mora, I., Cabrefiga, J. and Montesinos, E. 2011. Antimicrobial peptide genes in Bacillus strains from plant environments. International microbiology.14: 213-223. https://DOI: 10.2436/20.1501.01.151.
Ongena, M. and Philippe, J. 2008. Bacillus lipopeptides: versatile weapons for plant disease biocontrol. Trends Microbiology. 16:115-25. https://doi: 10.1016/j.tim.2007.12.009.
Parra, E., Bacab, I.M., Cristóbal, J., Tun J.M., y Ruíz, E. 2011. Patogenicidad de Fusarium solani (mart.) sacc. y Alternaria alternata (fries) keissler en thevetia peruviana (pers.) k. schum. y su control in vitro. Fitosanidad, 15: 231-236. https://www.redalyc.org/articulo.oa?id=209123682005.
Philibert, T., Lee, B.H. and Fabien, N. 2017. Current Status and New Perspectives on Chitin and Chitosan as Functional Biopolymers. Applied Biochemistry and Biotechnology. https://doi: 10.1007/s12010-016-2286-2.
Rodas, BA., Quero, M., Magaña, HF. and Reyes, A. 2009. Selección de cepas nativas con actividad Qui-tino-Proteolítica de Bacillus sp. aislados de suelos tropicales. Revista Colombiana de Biotecnología. 11: 107-113.
Sałek, K. and S. R. Euston. 2019. Sustainable microbial biosurfactants and bioemulsifiers for commercial exploitation. Process Biochemistry. 85: 143-155. https://doi.org/10.1016/j.procbio.2019.06.027.
Santovito, A., Gendusa, C., Ferraro, F., Musso, I., Costanzo M. and Piero, S.R,. 2018. Genomic damage induced by the widely used fungicide chlorothalonil in peripheral human lymphocytes. Ecotoxicology and Environmental Safety. 161: 578-583 https://doi.org/10.1016/j.ecoenv.2018.06.047.
Sosa, M; Ruiz E.; Mejía, M.; Reyes, A., Cristóbal, J.; Valencia, A. y Gutiérrez, O. 2012. Actividad antagónista in vitro de aislados de la clase bacilli de la península de Yucatán contra cuatro hongos fitopatógenos. Universidad y Ciencia. 28: 279-284. https://www.redalyc.org/articulo.oa?id=15425102007.
Trupo, M., Magarelli, R.A., Martino, M., Larocca, V., Giorgianni, Á. and Ambrico, A. 2023. Crude lipopeptides from culture of Bacillus subtilis strain ET-1 against Podosphaera xanthii on Cucumis melo. Journal of Natural Pesticide Research. 4: 100032. https://doi.org/10.1016/j.napere.2023.100032.
Yang, N., Wu, Q. and Xu, Y. 2020. Fe nanoparticles Enhanced Surfactin Production in Bacillus amyloliquefaciens. ACS Omega. 20: 6321-6329. https://doi: 10.1021/acsomega.9b03648.
Younes, I. and Rinaudo, M. 2015. Chitin and chitosan preparation from marine sources. Structure, prop-erties and applications. Marine Drugs. 2: 1133-1174. https://doi:10.3390/md13031133.
Zhu, H., Wu, S., Tang, S., Xu, J., He, Y., Ren, Z. and Liu, E. 2023. Isolation, identification and characterization of biopotential cyclic lipopeptides from Bacillus subtilis strain JN005 and its antifungal activity against rice pathogen Magnaporthe oryzae. Biological Control. 182: 105241. https://doi.org/10.1016/j.biocontrol.2023.105241.
Zhu, J., Zhang, L., Li, T., Ma, D., Gao, Y., Mu, W. and Liu, F.2018. Baseli ne sensitivity of Corynespora cassiicola to metconazole and efficacy of this fungicide. Crop Protection. 130: 105056. https://www.sciencedirect.com/science/article/pii/S0261219419304028.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
The journal Biotecnia is licensed under the Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0) license.




_(1)_(1).png)






_(2).jpg)





