Grape pomace on the growth performance and intestinal microbiota of finishing pigs
DOI:
https://doi.org/10.18633/biotecnia.v26.2177Palabras clave:
orujo de uva, microbiota intestinal, desempeño productivo, cerdosResumen
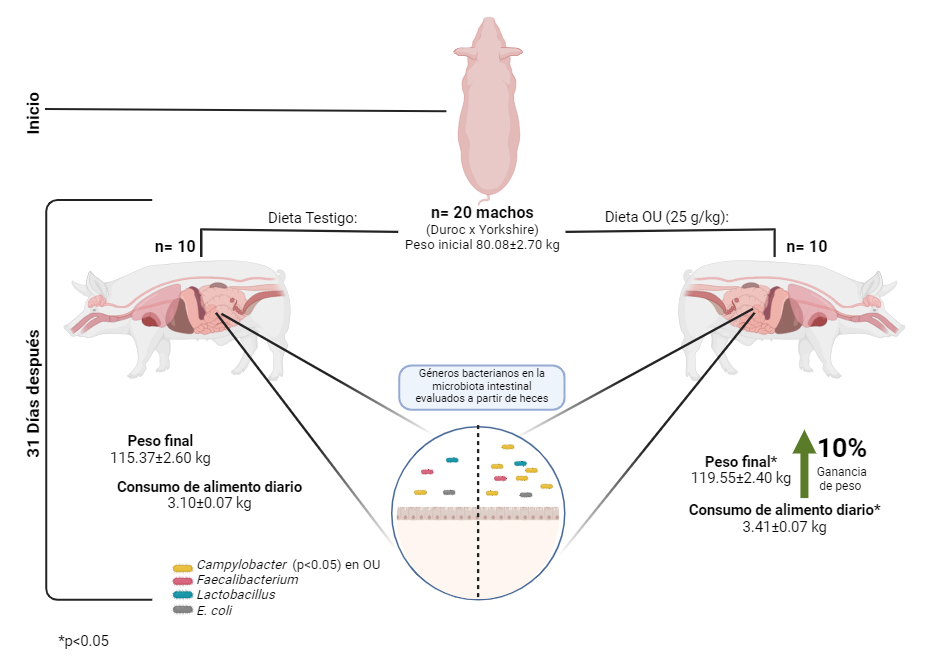
El objetivo del trabajo fue evaluar el efecto de la suplementación de orujo de uva (OU) sobre la microbiota intestinal (MI) y desempeño productivo de cerdos finalizadores. Se utilizaron 20 cerdos finalizadores machos (Duroc x Yorkshire, peso vivo inicial de 80 kg), alojados individualmente en corraletas provistas de bebedero y comedero. Se asignaron aleatoriamente a uno de dos tratamientos (n = 10): Testigo (dieta comercial, (DB) sin OU) y OU (DB + 25 g OU/ kg). La prueba de alimentación fue de 31 d. El comportamiento productivo se evaluó mediante la ganancia de peso diaria, el consumo de alimento diario y la conversión del alimento. Los cambios en la composición y abundancia en MI se evaluaron mediante qPCR en muestras de heces. La suplementación con OU incrementó significativamente (p < 0.05) el consumo de alimento y la ganancia de peso, pero no mostró efecto en la conversión alimenticia (p > 0.05). En la MI, la suplementación con OU no tuvo efecto (p > 0.05) en la abundancia de los géneros Lactobacillus spp, Faecalibacterium praustnitzi y E. coli, pero Campylobacter spp. incrementó (p < 0.05). Si bien, no se observó el comportamiento esperado en MI, su efecto positivo en la ganancia de peso podría permitir acortar los tiempos de producción.
Descargas
Citas
AOAC. 2005. Official method of Analysis. 18th Edition, Association of Officiating Analytical Chemists, Washington D.C.
Almeida, D., Machado, D., Andrade, J.C., Mendo, S., Gomes, A.M., Freitas, A.C. 2020. Evolving trends in next-generation probiotics: a 5W1H perspective. Critical Review in Food Science and Nutrition. 60(11):1783-1796.
Alter, T., Gaull F., Kasimir S., Gürtler M., Mielke H., Linnebur M., Fehlhaber K. 2005. Prevalences and transmission routes of Campylobacter spp. Strains within multiple pig farms. Veterinary Immunolo-gy. 108(3-4): 251-261.
Balbinoti, T.C.V., Stafussa, A.P., Haminiuk, C.W.I., Maciel, G.M., Sassaki, G.L., Jorge L.M.D.M. y Jorge R.M.M. 2020. Addition of grape pomace in the hydration step of parboiling increases the anti-oxidant properties of rice. International Journal of Food Science and Technology. 55(6): 2370-2380.
Bergamaschi, M., Tiezzi, F., Howard, J., Huang, J.Y., Kent, A. G. Schillebeeckx, C., McNulty, N. P., y Maltecca, C. 2020. Gut microbiome composition differences among breeds impact feed efficiency in swine. Microbiome 8 (110): 1-15
Bin, P., Tang, Z., Liu, S. Chen, S., Xia, Y., Liu, J., Wu, H., y Zhu, G. 2018. Intestinal microbiota mediates Enterotoxigenic Escherichia coli-induced diarrhea in piglets. BMC Veterinary Research 14(385): 1-13.
Broom, L. J. 2018. Gut barrier function: effects of (antibiotic) growth promoters on key barrier components and associations with growth performance. Poultry Science. 97(5): 1572-1578.
Carlson, M.S. y Fangman, T.J. 2018. Swine antibiotics and feed additives: food safety considerations. De-partment of Animal Sciences, University of Missouri-Columbia.
Casagrande, M., Zanela, J., Pereira, D., de Lima, V.A., Oldoni, T.L.C. y Carpes, S.T. 2019. Optimization of the extraction of antioxidant phenolic compounds from grape pomace using response surface methodology. Journal of Food Measurement and Characterization. 13(2):1120–1129.
Chedea, V.S., Palade, L.M., Marin, D.E., Pelmus, R.S., Habeanu, M., Rotar, M.C., Gras, M.A., Pistol, G.C. y Taranu I. 2018. Intestinal absorption and antioxidant activity of grape pomace polyphe-nols. Nutrients. 10(5): 588.
Choy, Y.Y., Quifer-Rada, P., Holstege, D.M., Frese S.A., Calvert,, C.C., Mills D.A., Lamuela-Raventos, R.M. y Waterhouse, A.L. 2014. Phenolic metabolites and substantial microbiome changes in pig fe-ces by ingesting grape seed proanthocyanidins. Food & Function. 5(9): 2298-2308.
CODEX. 1997. Límites máximos del codex para residuos de medicamentos veterinarios. [ Consultado 12 de Septiembre del 2022]. Disponible en: http:www.apps1.fao.org.
CORDIS. 2022. [Consultado 12 de Marzo 2023]. Disponible en: https://cordis.europa.eu/article/id/20620-regulation-bans-antibiotics-as-growth-promoters-in-animal-feed/es .
De Rodas, B., Youmans, B.P., Danzeisen, J.L., Tran, H. y Johnson, T.J. 2018. Microbiome profiling of commercial pigs from farrow to finish. Journal of Animal Science. 96(5): 1778-1794.
Echegaray, N., Munekata, P.E.S., Centeno, J.A., Pateiro, M., Carballo, J. y Lorenzo, J.M. 2021. Total phe-nol content and antioxidant activity of different celta pig carcass locations as affected by the finishing diet (chestnuts or commercial feed). Antioixidants. 10(5):1–19.
Fleckenstein, J.M., Hardwidge, P.R., Munson, G.P., Rasko, D.A. 2010. Sommerfelt H, Steinsland H. Mo-lecular mechanisms of enterotoxigenic Escherichia coli infection. Microbes & Infection. 12(2):89–98.
Furet, J.P., Firmesse, O., Gourmelon, M., Bridonneau, C., Tap, J., Mondot, S., Doré, J. y Corthier, G. 2009. Comparative assessment of human and farm animal faecal microbiota using real-time quantita-tive PCR. FEMS Microbiology Ecology. 68(3): 351-362.
Gardiner, G.E., Metzler-Zebeli, B.U., Lawlor, P.G. 2020. Impact of Intestinal Microbiota on Growth and Feed Efficiency in Pigs: A Review. Microorganisms. 28;8(12):1886.
Grosu, I. A., Pistol, G.C., Marin, D.E., Cismileanu, A., Palade. L.M., y Taranu I., 2020. Effects of dietary grape seed meal bioactive compounds on the colonic microbiota of weaned piglets with dextran sodi-um sulfate-induced colitis used as an inflammatory model. Frontier Veterinary Science. 7(31):1-14
Guevarra, R.B., Hong, S.H., Cho, J.H., Kim, B.R., Shin, J., Lee, J.H., Kang, B.N., Kim, Y.H., Wattana-phansak, S., Isaacson, R.E., Song, M. y Kim H.B. 2018. The dynamics of the piglet gut microbiome during the weaning transition in association with health and nutrition. Journal of Animal Science and Biotechnology. 9(1): 1-9.
Halmos, E.P., Christophersen, C.T., Bird, A.R., Shepherd, S.J., Gibson, P.R. y Muir J.G. 2015. Diets that differ in their FODMAP content alter the colonic luminal microenvironment. Gut. 64(1): 93-100.
Hao, R, Li, Q., Zhao, J., Li, H., Wang, W., Gao, J. 2015. Effects of grape seed procyanidins on growth performance, immune function and antioxidant capacity in weaned piglets. Livestock Science. 17: 237-242
Koh, A, De Vadder, F, Kovatcheva-Datchary, P., y Bäckhed F. 2016. From dietary fiber to host physiolo-gy: short-chain fatty acids as key bacterial metabolites. Cell. 65(6):1332-1345
Hintze, J. 2007. NCSS, PASS y GESS. Number Cruncher Statistical Systems. Kaysville, Utah.
Huijsdens, X.W., Linskens, R.K., Mak, M., Meuwissen, S.G., Vandenbroucke-Grauls, C.M. y Savelkoul P.H. 2002. Quantification of bacteria adherent to gastrointestinal mucosa by real-time PCR. Journal of Clinical Microbiology. 40(12)z. 4423-4427.
Kaevska,, M., Lorencova, A., Videnska, P., Sedlar, K., Provaznik, I. y Trckova M. 2016. Effect of sodium humate and zinc oxide used in prophylaxis of post-weaning diarrhoea on faecal microbiota composi-tion in weaned piglets. Veterinární Medicína. 61(6): 328-336.
Kafantaris, I., Stagos, D., Kotsampasi, B., Hatzis, A., Kypriotakis, A., Gerasopoulos, K., Makri, S., Goutzourelas N., Mitsagga, C., Giavasis, I., Petrotos, K., Kokkas,, S., Goulas P., Christodoulou, V. y Kouretas, D. 2018. Grape pomace improves performance, antioxidant status, fecal microbiota and meat quality of piglets. Animal. 12(2): 246-255.
Kim, H.B. y Isaacson, R.E. 2015. The pig gut microbial diversity: understanding the pig gut microbial ecol-ogy through the next generation high throughput sequencing. Veterinary Microbiology. 177(3-4): 242-251.
Kumanda, C., Mlambo, V. y Mnisi, C.M. 2019. From landfills to the dinner table: Red grape pomace waste as a nutraceutical for broiler chickens. Sustainability. 11(7): 1931.
Liu, G., Ren, W., Fang, J., C-AA, H., Guan, G., Al-Dhabi, N.A., Yin, J., Duraipandiyan, V., Chen, S., Peng, Y, et al. 2017. L-glutamine and l-arginine protect against enterotoxigenic Escherichia coli infec-tion via intestinal innate immunity in mice. Amino Acids. 49(12):1945–1954.
Livak, K.J. y Schmittgen, T. D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25(4): 402-408
Looft, T., Johnson T.A., Allen, H.K., Bayles,, D.O., Alt, D.P., Stedtfeld, R.D., Sul,W.J., Stedfeld, T.M., Chai, B., Cole, J.R., Hashsham, S.A., Tiedje, J.M. y Stanton, T.B. 2012. In-feed antibiotic effects on the swine intestinal microbiome. Proceedings of the National Academy of Sciences. 109(5): 1691-1696.
Luo, Y., Ren, W., Smidt, H., Wright, A.D.G., Yu, B., Schyns G., McCormack, U.M., Cowieson, A.J., Yu, J., He J., Yan, H., Wu J., Mackie, R.I. y Chen, D. 2022. Dynamic distribution of gut microbiota in pigs at different growth stages: composition and contribution. Microbiology Spectrum. 10(3): 1-15
McCormack, U. M., Curiao, T., Metzler-Zebeli, B. U., Magowan, E., Berry, D. P., Reyer, H., et al. (2019). Porcine feed efficiency-associated intestinal microbiota and physiological traits: finding consistent cross-locational biomarkers for residual feed intake. mSystems. 4(4):e00324-18
Massacci, F. R., Berri, M., Lemonnier, G., Guettier, E., Blanc, F., Jardet, D., y Estellé, J. 2020. Late wean-ing is associated with increased microbial diversity and Faecalibacterium prausnitzii abundance in the fecal microbiota of piglets. Animal Microbiome. 2(1):1-12.
Million, M., Angelakis, E., Paul, M., Armougom, F., Leibovici, L. y Raoult, D. 2012. Comparative meta-analysis of the effect of Lactobacillus species on weight gain in humans and animals. Microbial path-ogenesis. 53(2):100-108.
National Research Council (NRC) 2012. Nutrient Requirements of Swine: 11th Revised Edition. The Na-tional Academies Press. Washington, DC.
Niu Q, Li P, Hao S, Zhang Y, Kim SW, Li H, Ma, X., Gao, S., He, L., Wu, W., Huang, X., Hua, J., Zhou, B., y Huang, R., et al. 2015. Dynamic distribution of the gut microbiota and the relationship with ap-parent crude fiber digestibility and growth stages in pigs. Science. Report. 5:9938
Norma Oficial Mexicana NOM-051-ZOO-1995, trato humanitario en la movilización de animales. [Consultado 22 Marzo 2022]. Disponible en: http://dof.gob.mx/nota_detalle.php?codigo=4870842&fecha=23/03/1998
Park, Y.K., Ikegaki, M., Abreu, J.A. da S. y Alcici, N.M.F. 1998. Estudo da preparação dos extratos de própolis e suas aplicações. Food Science and Technology. 18(3): 313–318.
Pluske, J.R., Miller, D.W., Sterndale, S.O. y Turpin, D.L. 2019a. Associations between gastrointestinal-tract function and the stress response after weaning in pigs. Animal Production Science. 59(11): 2015-2022.
Pluske, J. R. y Zentek, J. 2019b. Gut nutrition and health in pigs and poultry. En Poultry and Pig Nutrition. Hendriks, W. H., Verstegen, M. W. A., y Babinszky, L (ed.), pp 77-95. Wageningen Academic Pub-lishers.
Pomar, C. y Remus, A. 2019. Precision pig feeding: a breakthrough toward sustainability. Animal Fron-tiers. 9(2): 52-59.
Quan, J., Wu Z, Ye Y, Peng L, Wu J, Ruan D, Qiu Y, Ding R, Wang X, Zheng E, Cai G, Huang W and Yang J .2020. Metagenomic characterization of intestinal regions in pigs with contrasting feed effi-ciency. Frontier Microbiology. 11(32):1-13
Rinttilä, T., Kassinen, A., Malinen, E., Krogius, L. y Palva, A. 2004. Development of an extensive set of 16S rDNA‐targeted primers for quantification of pathogenic and indigenous bacteria in faecal sam-ples by real‐time PCR. Journal of Applied Microbiology. 97(6): 1166-1177.
Sehm, J., Treutter, D. Lindermayer, H., Meyer, H.H.D., Pfaffl, M.W., 2011. The influence of apple- or red-grape pomace enriched piglet diet on blood parameters, bacterial colonisation, and marker gene expression in piglet white blood cells. Food Nutrition. Science. 2: 366-376
Singleton, V.L. y Rossi, J.A.J. 1965. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. American Journal of Enology and Vitivulture. 16: 44–168.
Taranu, I., Habeanu, M., Gras, M.A., Pistol, G.C., Lefter, N., Palade, M., Ropota, M., Sanda Chedea ,V., Marin, D.E. 2018. Assessment of the effect of grape seed cake inclusion in the diet of healthy fatten-ing-finishing pigs. Journal of Animal Physiology and Animal Nutrition. 102(1): e30-e42.
Taranu, I., Hermenean, A., Bulgaru, C., Pistol, G.C., Ciceu, A., Grosu, I.A. y Marin D.E. 2020. Diet con-taining grape seed meal by-product counteracts AFB1 toxicity in liver of pig after weaning. Ecotoxi-cology and Environmental Safety. 203: 110899.
Tayengwa, T., Chikwanha, O. C., Raffrenato, E., Dugan, M. E., Mutsvangwa, T., & Mapiye, C. 2021. Comparative effects of feeding citrus pulp and grape pomace on nutrient digestibility and utilization in steers. Animal, 15 (1): 100020.
Tayengwa, T., Chikwanha, O.C., Dugan, M.E.R., Mutsvangwa, T., Mapiye, C., 2020. Influence of feeding fruit by-products as alternative dietary fibre sources to wheat bran on beef production and quality of Angus steers. Meat Science. 161: 107969.
Torres-Pitarch, A., Gardiner, G.E., Cormican, P., Rea, M., Crispie, F., O'Doherty, J.V., Cozannet, P., Ryan, T., Cullen, J., Lawlor, P.G. 2020. Effect of cereal fermentation and carbohydrase supplementa-tion on growth, nutrient digestibility and intestinal microbiota in liquid-fed grow-finishing pigs. Sci-ence Reports. 10(1):13716.
Verhelst, R., Schroyen, M., Buys, N. y Niewold, T. 2014. Dietary polyphenols reduce diarrhea in entero-toxigenic Escherichia coli (ETEC) infected post-weaning piglets. Livestock Science. 160: 138-140.
Wang, R., Yu H., Fang, H., Jin, Y., Zhao, Y., Shen, J. y Zhang, J. 2020. Effects of dietary grape pomace on the intestinal microbiota and growth performance of weaned piglets. Archives of Animal Nutri-tion. 74(4): 296-308.
Williams, A.R., Krych, L., Fauzan Ahmad, H., Nejsum, P., Skovgaard, K., Nielsen, D.S. y Thamsborg, S.M. 2017. A polyphenol-enriched diet and Ascaris suum infection modulate mucosal immune re-sponses and gut microbiota composition in pigs. PLoS One. 12(10): e0186546.
World Health Organization. (2012). The evolving threat of antimicrobial resistance: options for action. World Health Organization. [Consultado 12 de abril del 2023]. Disponible en: https://apps.who.int/iris/handle/10665/44812.
Yang, H., Huang, X., Fang, S., Xin, W., Huang, L., y Chen, C. 2016. Uncovering the composition of mi-crobial community structure and metagenomics among three gut locations in pigs with distinct fatness. Science Reports 6:27427.
Yang, H., Huang X., Fang S., He M., Zhao Y., Wu Z., Yang M., Zhang Z., Chen C., y Huang L. 2017 Unraveling the fecal microbiota and metagenomic functional capacity associated with feed efficiency in pigs. Frontier Microbiology. 8:1555
Young, C.R., Harvey, R., Anderson,, R., Nisbet D., y Stanker, L.H. 2000. Enteric colonisation following natural exposure to Campylobacter in pigs. Research in Veterinary Science. 68(1): 75-78
Archivos adicionales
Publicado
Cómo citar
Número
Sección
Licencia
Derechos de autor 2023

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-CompartirIgual 4.0.
La revista Biotecnia se encuentra bajo la licencia Atribución-NoComercial-CompartirIgual 4.0 Internacional (CC BY-NC-SA 4.0)















_(2).jpg)








