Modelos murinos de diabetes para el avance de la administración oral de insulina: una revisión
DOI:
https://doi.org/10.18633/biotecnia.v27.2647Palabras clave:
Estudios preclínicos, biodisponibilidad, sistemas de liberación de fármacos, estreptozotocina, rata diabéticaResumen
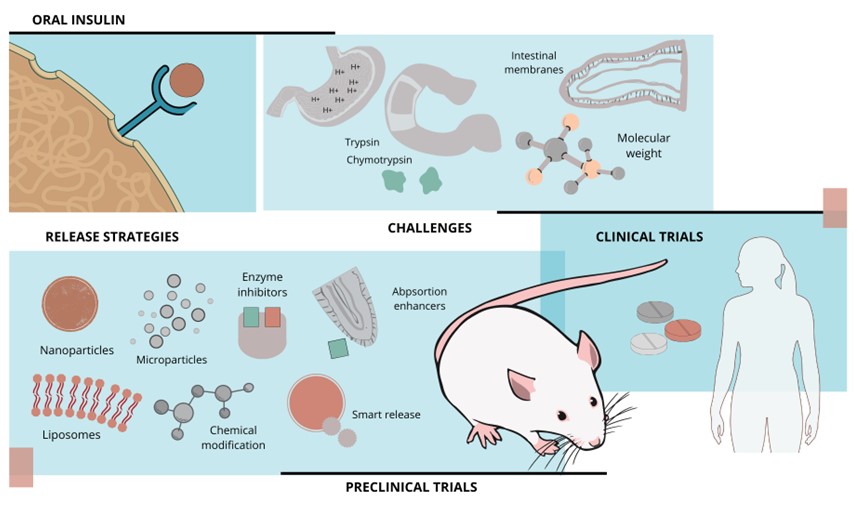
A pesar del descubrimiento de la insulina hace más de un siglo, su administración oral eficaz sigue siendo de alto interés en el campo de la investigación debido a su potencial de administración no invasiva. Esta revisión explora el papel fundamental de los modelos murinos de diabetes en el desarrollo de formulaciones de insulina oral efectivas. Se realizó una búsqueda bibliográfica en PubMed, ScienceDirect y Google Académico para identificar estudios experimentales publicados en los últimos cinco años. Los hallazgos revelan un progreso significativo en la mejora de la biodisponibilidad de la insulina oral y el control de la glucosa empleando técnicas innovadoras como nanopartículas, micropartículas, hidrogeles y sistemas de administración inteligentes. Estas técnicas se probaron en modelos animales de experimentación, predominantemente en ratas Wistar y Sprague Dawley con diabetes inducida por estreptozotocina. Sin embargo, trasladar estos avances a la práctica clínica en humanos sigue siendo un desafío. Optimizar los modelos experimentales y desarrollar tecnologías de administración sofisticadas es crucial para lograr terapias de insulina oral personalizadas y eficaces.
Descargas
Citas
Ahadian, S., Finbloom, J.A., Mofidfar, M., Diltemiz, S.E., Nasrollahi, F., Davoodi, E., Hosseini, V., Mylonaki, I., Sangabathuni, S., Montazerian, H., Fetah, K., Nasiri, R., Dokmeci, M.R., Stevens, M.M., Desai, T.A. and Khademhosseini, A. 2020. Micro and nanoscale technologies in oral drug delivery. Advanced Drug Delivery Reviews, 157: 37–62. https://doi.org/10.1016/j.addr.2020.07.012.
Alfa, J., Ben, A., Buxaderas, E., Akpa, P., Hanifah, A., Oseni, O.M.-L., Kenechukwu, F.C., Mumuni, M.A. and Diaz, D.D. 2024. Development and evaluation of PEG-gelatin-based microparticles to enhance the oral delivery of nsulin. Current Pharmaceutical Design, 30(24): 1939–1948. https://doi.org/10.2174/0113816128309449240527053640.
Alqahtani, M.S., Kazi, M., Alsenaidy, M.A. and Ahmad, M.Z. 2021. Advances in oral drug delivery. Frontiers in Pharmacology, 12. https://doi.org/10.3389/fphar.2021.618411.
American Diabetes Association 2024. 9. Pharmacologic approaches to glycemic treatment: standards of care in diabetes—2024. Diabetes Care, 47(Supplement_1), S158–S178. https://doi.org/10.2337/dc24-S009.
Amukarimi, S., Ramakrishna, S. and Mozafari, M. 2021. Smart biomaterials—A proposed definition and overview of the field. Current Opinion in Biomedical Engineering, 19: 100311. https://doi.org/10.1016/j.cobme.2021.100311.
Antar, S.A., Ashour, Nada A., Sharaky, M., Khattab, M., Ashour, Naira A., Zaid, R.T., Roh, E.J., Elkamhawy, A. and Al-Karmalawy, A.A. 2023. Diabetes mellitus: classification, mediators, and complications; A gate to identify potential targets for the development of new effective treatments. Biomedicine & Pharmacotherapy, 168: 115734. https://doi.org/10.1016/j.biopha.2023.115734.
Asal, H.A., Shoueir, K.R., El-Hagrasy, M.A. and Toson, E.A. 2022. Controlled synthesis of in-situ gold nanoparticles onto chitosan functionalized PLGA nanoparticles for oral insulin delivery. International Journal of Biological Macromolecules, 209: 2188–2196. https://doi.org/10.1016/j.ijbiomac.2022.04.200.
Athmuri, D.N. and Shiekh, P.A. 2023. Experimental diabetic animal models to study diabetes and diabetic complications. MethodsX, 11: 102474. https://doi.org/10.1016/j.mex.2023.102474.
Bashyal, S., Noh, G., Keum, T., Choi, Y.W. and Lee, S. 2016. Cell penetrating peptides as an innovative approach for drug delivery; then, present and the future. Journal of Pharmaceutical Investigation, 46(3): 205–220. https://doi.org/10.1007/s40005-016-0253-0.
Beijing Hospital. 2021. A etudy evaluating the bioavailability of oral insulin (N11005). ClinicalTrials.gov. https://clinicaltrials.gov/study/NCT04975022?intr=N11005&rank=1
Bolli, G.B., Porcellati, F., Lucidi, P. and Fanelli, C.G. 2021. The physiological basis of insulin therapy in people with diabetes mellitus. Diabetes Research and Clinical Practice, 175: 108839. https://doi.org/10.1016/j.diabres.2021.108839.
Bolli, G.B., Porcellati, F., Lucidi, P., Fanelli, C.G. and Owens, D.R. 2022. One-hundred year evolution of prandial insulin preparations: from animal pancreas extracts to rapid-acting analogs. Metabolism, 126: 154935. https://doi.org/10.1016/J.METABOL.2021.154935.
Boppana, S.H., Kutikuppala, L.V.S., Sharma, S., C, M., Rangari, G., Misra, A.K., Kandi, V., Mishra, S., Singh, P.K., Rabaan, A.A., Mohapatra, R.K. and Kudrat‐E‐Zahan, Md. 2024. Current approaches in smart nano‐inspired drug delivery: a narrative review. Health Science Reports, 7(4). https://doi.org/10.1002/hsr2.2065.
Bordbar-Khiabani, A. and Gasik, M. 2022. Smart hydrogels for advanced drug delivery systems. International Journal of Molecular Sciences, 23(7): 3665. https://doi.org/10.3390/ijms23073665.
Bryda, E.C. 2013. The Mighty Mouse: the impact of rodents on advances in biomedical research. Missouri medicine, 110(3): 207–11.
Cao, S.-J., Xu, S., Wang, H.-M., Ling, Y., Dong, J., Xia, R.-D. and Sun, X.-H. 2019. Nanoparticles: oral delivery for protein and peptide drugs. AAPS PharmSciTech, 20(5): 190. https://doi.org/10.1208/s12249-019-1325-z.
Chamsai, B., Opanasopit, P. and Samprasit, W. 2023. Fast disintegrating dosage forms of mucoadhesive-based nanoparticles for oral insulin delivery: optimization to in vivo evaluation. International Journal of Pharmaceutics, 647: 123513. https://doi.org/10.1016/j.ijpharm.2023.123513.
Chircov, C. and Grumezescu, A.M. 2022. Microelectromechanical Systems (MEMS) for biomedical applications. Micromachines, 13(2): 164. https://doi.org/10.3390/mi13020164.
Chouhan, R., Goswami, S. and Bajpai, A.K. 2017. Recent advancements in oral delivery of insulin: from challenges to solutions. Nanostructures for Oral Medicine, 435–465. https://doi.org/10.1016/B978-0-323-47720-8.00016-X.
Chu, C., Deng, Y., Liu, H., Wei, M., Xu, X., Gou, J., He, H., Yin, T., Zhang, Y. and Tang, X. 2023. In situ rearranged multifunctional lipid nanoparticles via synergistic potentiation for oral insulin delivery. International Journal of Pharmaceutics, 636: 122811. https://doi.org/10.1016/j.ijpharm.2023.122811.
Dağaşan, S. and Erbaş, O. 2021. Insulin structure, function and diabetes models in animals. Journal of Experimental and Basic Medical Sciences, 1(3): 96–101. https://doi.org/10.5606/jebms.2020.75622.
Dan, N., Samanta, K. and Almoazen, H. 2020. An update on pharmaceutical strategies for oral delivery of therapeutic peptides and proteins in adults and pediatrics. Children, 7(12): 307. https://doi.org/10.3390/children7120307.
Daniell, H., Singh, R., Mangu, V., Nair, S.K., Wakade, G. and Balashova, N. 2023. Affordable oral proinsulin bioencapsulated in plant cells regulates blood sugar levels similar to natural insulin. Biomaterials, 298: 122142. https://doi.org/10.1016/j.biomaterials.2023.122142.
Demir, G., Er, E., Atik Altınok, Y., Özen, S., Darcan, Ş. and Gökşen, D. 2022. Local complications of insulin administration sites and effect on diabetes management. Journal of Clinical Nursing, 31(17–18): 2530–2538. https://doi.org/10.1111/jocn.16071.
Denayer, T., Stöhr, T. and Roy, M. Van. 2014. Animal models in translational medicine: validation and prediction. European Journal of Molecular & Clinical Medicine, 2(1): 5. https://doi.org/10.1016/j.nhtm.2014.08.001.
Eldor, R., Francis, B.H., Fleming, A., Neutel, J., Homer, K., Kidron, M. and Rosenstock, J. 2023. Oral insulin (ORMD‐0801) in type 2 diabetes mellitus: a dose‐finding 12‐week randomized placebo‐controlled study. Diabetes, Obesity and Metabolism, 25(4): 943–952. https://doi.org/10.1111/dom.14901.
Elema, B., Degu, T. and Fesseha, H. 2020. Smart drug delivery system: a review. Archives of Clinical Case Reports, 1(1): 11–16.
Fujiwara, S. 2018. Humanized mice: a brief overview on their diverse applications in biomedical research. Journal of Cellular Physiology, 233(4): 2889–2901. https://doi.org/10.1002/jcp.26022.
Furman, B.L. 2021. Streptozotocin‐induced diabetic models in mice and rats. Current Protocols, 1(4). https://doi.org/10.1002/cpz1.78.
Goo, Y.T., Lee, S., Choi, J.Y., Kim, M.S., Sin, G.H., Hong, S.H., Kim, C.H., Song, S.H. and Choi, Y.W. 2022. Enhanced oral absorption of insulin: hydrophobic ion pairing and a self-microemulsifying drug delivery system using a D-optimal mixture design. Drug Delivery, 29(1): 2831–2845. https://doi.org/10.1080/10717544.2022.2118399.
Halberg, I.B., Lyby, K., Wassermann, K., Heise, T., Zijlstra, E. and Plum-Mörschel, L. 2019. Efficacy and safety of oral basal insulin versus subcutaneous insulin glargine in type 2 diabetes: a randomised, double-blind, phase 2 trial. The Lancet Diabetes & Endocrinology, 7(3): 179–188. https://doi.org/10.1016/S2213-8587(18)30372-3.
He, H., Lu, Y., Qi, J., Zhu, Q., Chen, Z. and Wu, W. 2019. Adapting liposomes for oral drug delivery. Acta Pharmaceutica Sinica B, 9(1): 36–48. https://doi.org/10.1016/j.apsb.2018.06.005.
Heise, T., Plum‐Mörschel, L. and Zijlstra, E. 2023. Oral insulin: a history of ambition, failure and data torturing. Diabetes, Obesity and Metabolism, 25(4): 940–942. https://doi.org/10.1111/dom.14984.
Homayun, B., Lin, X. and Choi, H.-J. 2019. Challenges and recent progress in oral drug delivery systems for biopharmaceuticals. Pharmaceutics, 11(3): 129. https://doi.org/10.3390/pharmaceutics11030129.
International Diabetes Federation. 2025. IDF Diabetes Atlas 2025 11th edition. https://diabetesatlas.org/resources/idf-diabetes-atlas-2025/
Ioannidis, J.P.A., Kim, B.Y.S. and Trounson, A. 2018. How to design preclinical studies in nanomedicine and cell therapy to maximize the prospects of clinical translation. Nature Biomedical Engineering, 2(11): 797–809. https://doi.org/10.1038/s41551-018-0314-y.
Ito, S., Torii, Y., Chikamatsu, S., Harada, T., Yamaguchi, S., Ogata, S., Sonoda, K., Wakayama, T., Masuda, T. and Ohtsuki, S. 2021. Oral coadministration of Zn-Insulin with d-form small intestine-permeable cyclic peptide enhances its blood glucose-lowering effect in mice. Molecular Pharmaceutics, 18(4): 1593–1603. https://doi.org/10.1021/acs.molpharmaceut.0c01010.
Janapati, Y.K. and Junapudi, S. 2024. Progress in experimental models to investigate the in vivo and in vitro antidiabetic activity of drugs. Animal Models and Experimental Medicine, 7(3): 297–309. https://doi.org/10.1002/ame2.12442.
Kapitza, C., Zijlstra, E., Heinemann, L., Castelli, M.C., Riley, G. and Heise, T. 2010. Oral insulin: a comparison with subcutaneous regular human insulin in patients with type 2 diabetes. Diabetes Care, 33(6): 1288–1290. https://doi.org/10.2337/dc09-1807.
Karamanou, M., Protogerou, A., Tsoucalas, G., Androutsos, G. and Poulakou-Rebelakou, E. 2016. Milestones in the history of diabetes mellitus: the main contributors. World Journal of Diabetes, 7(1): 1. https://doi.org/10.4239/wjd.v7.i1.1.
Khedkar, A., Lebovitz, H., Fleming, A., Cherrington, A., Jose, V., Athalye, S.N. and Vishweswaramurthy, A. 2019. Impact of insulin Tregopil and its permeation enhancer on pharmacokinetics of metformin in healthy volunteers: randomized, open‐label, placebo‐controlled, crossover study. Clinical and Translational Science, 12(3): 276–282. https://doi.org/10.1111/cts.12609.
Korivi, M., Huang, Y.-W. and Liu, B.R. 2021. Cell-penetrating peptides as a potential drug delivery system for effective treatment of diabetes. Current Pharmaceutical Design, 27(6): 816–825. https://doi.org/10.2174/1381612826666201019102640.
Kottaisamy, C.P.D., Raj, D.S., Prasanth Kumar, V. and Sankaran, U. 2021. Experimental animal models for diabetes and its related complications—a review. Laboratory Animal Research, 37(1): 23. https://doi.org/10.1186/s42826-021-00101-4.
Kramer, C.K., Retnakaran, R. and Zinman, B. 2021. Insulin and insulin analogs as antidiabetic therapy: a perspective from clinical trials. Cell Metabolism, 33(4): 740–747. https://doi.org/10.1016/j.cmet.2021.03.014.
Kristensen, M. and Nielsen, H.M. 2016. Cell‐penetrating peptides as carriers for oral delivery of biopharmaceuticals’, Basic & Clinical Pharmacology & Toxicology, 118(2): 99–106. https://doi.org/10.1111/bcpt.12515.
Lebovitz, H.E., Fleming, A., Cherrington, A.D., Joshi, S., Athalye, S.N., Loganathan, S., Vishweswaramurthy, A., Panda, J. and Marwah, A. 2022. Efficacy and safety of Tregopil, a novel, ultra-rapid acting oral prandial insulin analog, as part of a basal-bolus regimen in type 2 diabetes: a randomized, active-controlled phase 2/3 study’, Expert Opinion on Pharmacotherapy, 23(16): 1855–1863. https://doi.org/10.1080/14656566.2022.2141569.
Li, M., Wang, N., Liu, R., Zhang, X., He, W., Zhang, W., Li, J., Peng, C. and Li, Y. 2024. pH and H2O2 dual-sensitive nanoparticles enable enhanced and safe glucose-responsive oral insulin delivery for diabetes mellitus treatment. Theranostics, 14(14): 5596–5607. https://doi.org/10.7150/thno.98177.
López Soto, L.F., Candia Plata, C., Reyes Márquez, V., Arredondo Damián, J., Mata Pineda, A.L., Álvarez Hernández, G., Lorenzana Basaldúa, R. and Soto Guzman, A. 2024. Modelos murinos de diabetes para el estudio de compuestos bioactivos. TECNOCIENCIA Chihuahua, 18(1): e1402. https://doi.org/10.54167/tch.v18i1.1402.
Lu, X., Xie, Q., Pan, X., Zhang, R., Zhang, X., Peng, G., Zhang, Y., Shen, S. and Tong, N. 2024. Type 2 diabetes mellitus in adults: pathogenesis, prevention and therapy. Signal Transduction and Targeted Therapy, 9(1): 262. https://doi.org/10.1038/s41392-024-01951-9.
Ma, C., Peng, Y., Li, H. and Chen, W. 2021. Organ-on-a-Chip: a new paradigm for drug development. Trends in Pharmacological Sciences, 42(2): 119–133. https://doi.org/10.1016/j.tips.2020.11.009.
Mak, I.W., Evaniew, N. and Ghert, M. 2014. Lost in translation: animal models and clinical trials in cancer treatment. American journal of translational research, 6(2): 114–8.
Martín-Carro, B., Donate-Correa, J., Fernández-Villabrille, S., Martín-Vírgala, J., Panizo, S., Carrillo-López, N., Martínez-Arias, L., Navarro-González, J.F., Naves-Díaz, M., Fernández-Martín, J.L., Alonso-Montes, C. and Cannata-Andía, J.B. 2023. Experimental models to study diabetes mellitus and its complications: limitations and new opportunities. International Journal of Molecular Sciences, 24(12): 10309. https://doi.org/10.3390/ijms241210309.
McGonigle, P. and Ruggeri, B. 2014. Animal models of human disease:cChallenges in enabling translation. Biochemical Pharmacology, 87(1): 162–171. https://doi.org/10.1016/j.bcp.2013.08.006.
Morales-Burgos, A.M., Carvajal-Millan, E., Sotelo-Cruz, N., Rascón-Chu, A., Lizardi-Mendoza, J., López-Franco, Y.L., Martínez-Porchas, M. and Canett-Romero, R. 2021. Highly cross-linked arabinoxylans microspheres as a microbiota-activated carrier for colon-specific insulin delivery. European Journal of Pharmaceutics and Biopharmaceutics, 163: 16–22. https://doi.org/10.1016/j.ejpb.2021.02.014.
Mudassir, J., Darwis, Y., Muhamad, S. and Ali Khan, A. 2019. Self-assembled insulin and nanogels polyelectrolyte complex (Ins/NGs-PEC) for oral insulin delivery: characterization, lyophilization and in-vivo evaluation. International Journal of Nanomedicine, 14: 4895–4909. https://doi.org/10.2147/IJN.S199507.
Muheem, A., Shakeel, F., Jahangir, M.A., Anwar, M., Mallick, N., Jain, G.K., Warsi, M.H. and Ahmad, F.J. 2016. A review on the strategies for oral delivery of proteins and peptides and their clinical perspectives. Saudi Pharmaceutical Journal, 24(4): 413–428. https://doi.org/10.1016/j.jsps.2014.06.004.
Mumuni, M.A., Kenechukwu, F.C., Ofokansi, K.C., Attama, A.A. and Díaz, D.D. 2020. Insulin-loaded mucoadhesive nanoparticles based on mucin-chitosan complexes for oral delivery and diabetes treatment. Carbohydrate Polymers, 229: 115506. https://doi.org/10.1016/J.CARBPOL.2019.115506.
Muntoni, E., Anfossi, L., Milla, P., Marini, E., Ferraris, C., Capucchio, M.T., Colombino, E., Segale, L., Porta, M. and Battaglia, L. 2021. Glargine insulin loaded lipid nanoparticles: oral delivery of liquid and solid oral dosage forms. Nutrition, Metabolism and Cardiovascular Diseases, 31(2): 691–698. https://doi.org/10.1016/j.numecd.2020.09.020.
Nabi-Afjadi, M., Ostadhadi, S., Liaghat, M., Pasupulla, A.P., Masoumi, S., Aziziyan, F., Zalpoor, H., Abkhooie, L. and Tarhriz, V. 2024. Revolutionizing type 1 diabetes management: exploring oral insulin and adjunctive treatments. Biomedicine & Pharmacotherapy, 176: 116808. https://doi.org/10.1016/j.biopha.2024.116808.
New, R.R.C., Ramanujam, S., Chaudhari, V., Bogus, M., Travers, G.N. and Namjoshi, G. 2023. Safety and efficacy of an oral insulin (Capsulin) in patients with early‐stage type 2 diabetes: a dose‐ranging phase 2b study. Diabetes, Obesity and Metabolism, 25(4): 953–960. https://doi.org/10.1111/dom.14922.
Nicze, M., Borówka, M., Dec, A., Niemiec, A., Bułdak, Ł. and Okopień, B. 2024. The current and promising oral delivery methods for protein- and peptide-based drugs. International Journal of Molecular Sciences, 25(2): 815. https://doi.org/10.3390/ijms25020815.
Novo Nordisk A/S. 2017. A two part trial investigating NN1952 in healthy subjects and subjects with type 1 and 3ype 2 diabetes. ClinicalTrial.gov. https://clinicaltrials.gov/study/NCT01028404?intr=NN1952%20&rank=1
Ojo, O.A., Ibrahim, H.S., Rotimi, D.E., Ogunlakin, A.D. and Ojo, A.B. 2023. Diabetes mellitus: from molecular mechanism to pathophysiology and pharmacology. Medicine in Novel Technology and Devices, 19: 100247. https://doi.org/10.1016/j.medntd.2023.100247.
Ong, K.L., Stafford, L.K., McLaughlin, S.A., Boyko, E.J., Vollset, S.E., Smith, A.E et al. 2023. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. The Lancet, 402(10397): 203–234. https://doi.org/10.1016/S0140-6736(23)01301-6.
Oramed, Ltd. 2025. A phase 3 study to evaluate the efficacy and safety of ORMD-0801 in subjects with type 2 diabetes mellitus. ClinicalTrial.gov. https://clinicaltrials.gov/study/NCT04754334?intr=ORMD-0801&rank=5
Pan, Q., Wang, X., Li, W., Chen, X., Zhuang, Y., Zhou, Q., Huang, Y., Zhou, Y., Lan, L., Wang, Z., Wang, W., Hong, J., Hao, W.-H., Yang, Y.-T. and Guo, L. 2023. Pharmacokinetics, pharmacodynamics, and safety of prandial oral insulin (N11005) in healthy subjects. Frontiers in Endocrinology, 14. https://doi.org/10.3389/fendo.2023.1172327.
Pandey, S., Chmelir, T. and Chottova Dvorakova, M. 2023. Animal models in diabetic research—History, presence, and future perspectives. Biomedicines, 11(10): 2852. https://doi.org/10.3390/biomedicines11102852.
Pang, H., Wu, Y., Chen, Y., Chen, C., Nie, X., Li, P., Huang, G., Xu, Z.P. and Han, F.Y. 2024. Development of polysaccharide-coated layered double hydroxide nanocomposites for enhanced oral insulin delivery. Drug Delivery and Translational Research, 14(9): 2345–2355. https://doi.org/10.1007/s13346-023-01504-7.
Peng, H., Wang, J., Chen, J., Peng, Y., Wang, X., Chen, Y., Kaplan, D.L. and Wang, Q. 2023. Challenges and opportunities in delivering oral peptides and proteins. Expert Opinion on Drug Delivery, 20(10): 1349–1369. https://doi.org/10.1080/17425247.2023.2237408.
Raguraman, V., Jayasri, M.A. and Suthindhiran, K. 2020. Magnetosome mediated oral insulin delivery and its possible use in diabetes management. Journal of Materials Science: Materials in Medicine, 31(8): 75. https://doi.org/10.1007/s10856-020-06417-2.
Rahman, M.S., Hossain, K.S., Das, S., Kundu, S., Adegoke, E.O., Rahman, Md.A., Hannan, Md.A., Uddin, M.J. and Pang, M.-G. 2021. Role of insulin in health and disease: an update. International Journal of Molecular Sciences, 22(12): 6403. https://doi.org/10.3390/ijms22126403.
Rehmani, S., McLaughlin, C.M., Eltaher, H.M., Moffett, R.C., Flatt, P.R. and Dixon, J.E. 2023. Orally-delivered insulin-peptide nanocomplexes enhance transcytosis from cellular depots and improve diabetic blood glucose control. Journal of Controlled Release, 360: 93–109. https://doi.org/10.1016/j.jconrel.2023.06.006.
Ren, C., Zhong, D., Qi, Y., Liu, C., Liu, X., Chen, S., Yan, S. and Zhou, M. 2023. Bioinspired pH-responsive microalgal hydrogels for oral insulin delivery with both hypoglycemic and insulin sensitizing effects. ACS Nano, 17(14): 14161–14175. https://doi.org/10.1021/acsnano.3c04897.
Rodbard, H.W. and Rodbard, D. 2020. Biosynthetic human insulin and insulin analogs. American Journal of Therapeutics, 27(1): e42–e51. https://doi.org/10.1097/MJT.0000000000001089.
Sahu, T., Ratre, Y.K., Chauhan, S., Bhaskar, L.V.K.S., Nair, M.P. and Verma, H.K. 2021. Nanotechnology based drug delivery system: current strategies and emerging therapeutic potential for medical science. Journal of Drug Delivery Science and Technology, 63: 102487. https://doi.org/10.1016/j.jddst.2021.102487.
Shapira‐Furman, T. and Domb, A.J. 2023. Insulin extended release from PLA‐PEG stereocomplex nanoparticles. Macromolecular Bioscience, 24(5). https://doi.org/10.1002/mabi.202300497.
Sharma, A.K., Taneja, G., Kumar, Ashish, Sahu, M., Sharma, G., Kumar, Asim, Sardana, S. and Deep, A. 2019. Insulin analogs: glimpse on contemporary facts and future prospective. Life Sciences, 219: 90–99. https://doi.org/10.1016/j.lfs.2019.01.011.
Sharma, P., Garg, A., Garg, S. and Singh, V. 2016. Animal model used for experimental study of diabetes mellitus: an overview’, Asian Journal of Biomaterial Research, 2(4): 99–110.
Sharmah, B., Barman, H., Afzal, N.U., Loying, R., Kabir, M.E., Borah, A., Das, J., Kalita, J. and Manna, P. 2024. Surface-functionalized nanoceria: dual action in diabetes management via glucose-responsive insulin delivery and oxidative stress mitigation. ACS Biomaterials Science & Engineering, 10(10): 6397–6414. https://doi.org/10.1021/acsbiomaterials.4c01368.
Spain, C.V., Wright, J.J., Hahn, R.M., Wivel, A. and Martin, A.A. 2016. Self-reported barriers to adherence and persistence to treatment with injectable medications for type 2 diabetes. Clinical Therapeutics, 38(7): 1653-1664.e1. https://doi.org/10.1016/j.clinthera.2016.05.009.
Su, F.Y., Lin, K.J., Sonaje, K., Wey, S.P., Yen, T.C., Ho, Y.C., Panda, N., Chuang, E.Y., Maiti, B. and Sung, H.W. 2012. Protease inhibition and absorption enhancement by functional nanoparticles for effective oral insulin delivery. Biomaterials, 33(9): 2801–2811. https://doi.org/10.1016/J.BIOMATERIALS.2011.12.038.
Sultana, A., Zare, M., Thomas, V., Kumar, T.S.S. and Ramakrishna, S. 2022. Nano-based drug delivery systems: conventional drug delivery routes, recent developments and future prospects. Medicine in Drug Discovery, 15: 100134. https://doi.org/10.1016/j.medidd.2022.100134.
Sun, J., Yang, Z. and Teng, L. 2020. Nanotechnology and microtechnology in drug delivery systems. Dose-Response, 18(2): 55932582090781. https://doi.org/10.1177/1559325820907810.
Verma, S., Goand, U.K., Husain, A., Katekar, R.A., Garg, R. and Gayen, J.R. 2021. Challenges of peptide and protein drug delivery by oral route: current strategies to improve the bioavailability. Drug Development Research, 82(7): 927–944. https://doi.org/10.1002/ddr.21832.
Wang, A., Yang, T., Fan, W., Yang, Y., Zhu, Q., Guo, S., Zhu, C., Yuan, Y., Zhang, T. and Gan, Y. 2019. Protein corona liposomes achieve efficient oral insulin delivery by overcoming mucus and epithelial barriers. Advanced Healthcare Materials, 8(12). https://doi.org/10.1002/adhm.201801123.
Wang, J., Wang, Z., Yu, J., Kahkoska, A.R., Buse, J.B. and Gu, Z. 2020. Glucose‐responsive insulin and delivery dystems: innovation and translation. Advanced Materials, 32(13). https://doi.org/10.1002/adma.201902004.
Wong, C.Y., Al-Salami, H. and Dass, C.R. 2018. Microparticles, microcapsules and microspheres: a review of recent developments and prospects for oral delivery of insulin. International Journal of Pharmaceutics, 537(1–2): 223–244. https://doi.org/10.1016/j.ijpharm.2017.12.036.
Wong, C.Y., Martinez, J. and Dass, C.R. 2016. Oral delivery of insulin for treatment of diabetes: status quo, challenges and opportunities. Journal of Pharmacy and Pharmacology, 68(9): 1093–1108. https://doi.org/10.1111/jphp.12607.
Wysoczański, B., Świątek, M. and Wójcik-Gładysz, A. 2024. Organ-on-a-Chip models—New possibilities in experimental science and disease modeling. Biomolecules, 14(12): 1569. https://doi.org/10.3390/biom14121569.
Xiao, Y., Tang, Z., Wang, J., Liu, C., Kong, N., Farokhzad, O.C. and Tao, W. 2020. Oral insulin delivery platforms: strategies to address the biological barriers. Angewandte Chemie International Edition, 59(45): 19787–19795. https://doi.org/10.1002/anie.202008879.
Xu, M., Huang, J., Jiang, S., He, J., Wang, Z., Qin, H. and Guan, Y.-Q. 2022. Glucose sensitive konjac glucomannan/concanavalin A nanoparticles as oral insulin delivery system. International Journal of Biological Macromolecules, 202: 296–308. https://doi.org/10.1016/j.ijbiomac.2022.01.048.
Xu, Y., Shrestha, N., Préat, V. and Beloqui, A. 2020. Overcoming the intestinal barrier: a look into targeting approaches for improved oral drug delivery systems. Journal of Controlled Release, 322: 486–508. https://doi.org/10.1016/j.jconrel.2020.04.006.
Yang, Y., Chen, S., Liu, Y., Huang, Y., Cheong, K.-L., Teng, B. and Liu, W. 2021. Long-term treatment of polysaccharides-based hydrogel microparticles as oral insulin delivery in streptozotocin-induced type 2 diabetic mice. Biomedicine & Pharmacotherapy, 133: 110941. https://doi.org/10.1016/j.biopha.2020.110941.
Zhang, E., Zhu, H., Song, B., Shi, Y. and Cao, Z. 2024. Recent advances in oral insulin delivery technologies. Journal of Controlled Release, 366: 221–230. https://doi.org/10.1016/j.jconrel.2023.12.045.
Zhang, F., Pei, X., Peng, X., Gou, D., Fan, X., Zheng, X., Song, C., Zhou, Y. and Cui, S. 2022. Dual crosslinking of folic acid-modified pectin nanoparticles for enhanced oral insulin delivery. Biomaterials Advances, 135: 212746. https://doi.org/10.1016/j.bioadv.2022.212746.
Zhang, H., Gu, Z., Li, W., Guo, Lili, Wang, L., Guo, Lan, Ma, S., Han, B. and Chang, J. 2022. pH-sensitive O-carboxymethyl chitosan/sodium alginate nanohydrogel for enhanced oral delivery of insulin. International Journal of Biological Macromolecules, 223: 433–445.: https://doi.org/10.1016/j.ijbiomac.2022.10.274.
Zhou, B., Rayner, A.W., Gregg, E.W., Sheffer, K.E., Carrillo-Larco, R.M., Bennett, J.E. et al. 2024. Worldwide trends in diabetes prevalence and treatment from 1990 to 2022: a pooled analysis of 1108 population-representative studies with 141 million participants. The Lancet, 404(10467): 2077–2093. https://doi.org/10.1016/S0140-6736(24)02317-1
Zhou, J., Ma, H., Guan, M., Feng, J., Dong, X., Wei, Y. and Zhang, T. 2024. Anti-inflammatory Fucoidan-ConA oral insulin nanosystems for smart blood glucose regulation. International Journal of Pharmaceutics, 659: 124250. https://doi.org/10.1016/j.ijpharm.2024.124250.
Zhou, Y., Liu, L., Cao, Y., Yu, S., He, C. and Chen, X. 2020. A nanocomposite vehicle based on metal–organic framework nanoparticle incorporated biodegradable microspheres for enhanced oral insulin delivery. ACS Applied Materials & Interfaces, 12(20): 22581–22592. https://doi.org/10.1021/acsami.0c04303.
Zijlstra, E., Heinemann, L. and Plum-Mörschel, L. 2014. Oral insulin reloaded. Journal of Diabetes Science and Technology, 8(3): 458–465. https://doi.org/10.1177/1932296814529988.
Descargas
Publicado
Cómo citar
Número
Sección
Licencia
Derechos de autor 2025

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-CompartirIgual 4.0.
La revista Biotecnia se encuentra bajo la licencia Atribución-NoComercial-CompartirIgual 4.0 Internacional (CC BY-NC-SA 4.0)















_(2).jpg)








