A simple solution method to prepare VO2:Co2+ precursors for thin film deposition by solution-processing method
A simple solution method to prepare VO2:Co2+
DOI:
https://doi.org/10.18633/biotecnia.v25i2.1886Keywords:
cobalt doped vanadium oxide, spin-coated thin films, porous morphology, solution processing without ligands.Abstract
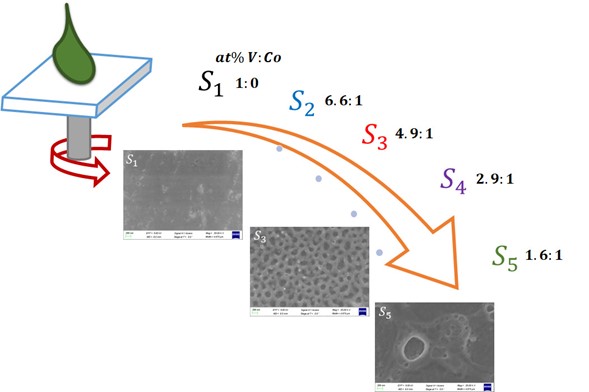
Solution-processing is a low-cost solution method to preparea variety of organic or inorganic thin films. For metal oxide compounds, a solution-processing solution of an organometallic compound is frequently used as a precursor to be spin coated, followed by a thermal annealing to form metal oxide. In this work, vanadium oxide powders are obtained from a simple acid-base reaction, and then they are dispersed in isopropyl alcohol to form a solution for spin-coating. Different amount of cobalt salt are also added together with VOx into isopropyl alcohol to form VOx:Co2+ solutions. After thermal annealing at 200 °C, continuous transparent thin films are obtained. Optical, structural, morphological and chemical binding energies of those films are analyzed. It is found that amorphous VO2:Co2+ compound is formed in those films with V:Co atomic ratios between 6.6:1 and 1.6:1. Optical absorption onsets of those films are around 2.3 eV. An interesting interconnected porous morphology is observed when the atomic ratio of V:Co is around 4.9:1. It is concluded that porous amorphous cobalt doped vanadium oxide thin films can be obtained from a spin-coating process at low annealing temperature from a simple solution without any complex agent.
Downloads
References
A. Herera-Gomez (no date) AAnalyzer a peak-fitting program for photoemission data. Available at: http://rdataa.com/aanalyzer/aanaHome.htm (Accessed: 18 April 2020).
Bae, J. W., Koo, B. R. and Ahn, H. J. (2019) ‘Fe doping effect of vanadium oxide films for enhanced switching electrochromic performances’, Ceramics International, 45(6), pp. 7137–7142. doi: 10.1016/j.ceramint.2018.12.219.
Biesinger, M. C. et al. (2011) ‘Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni’, Applied Surface Science, 257(7), pp. 2717–2730. doi: 10.1016/j.apsusc.2010.10.051.
Cabrera-German, D., Gomez-Sosa, G. and Herrera-Gomez, A. (2016) ‘Accurate peak fitting and subsequent quantitative composition analysis of the spectrum of Co 2p obtained with Al Kα radiation: I: cobalt spinel’, Surface and Interface Analysis, 48(5), pp. 252–256. doi: 10.1002/sia.5933.
Channu, V. S. R. et al. (2011) ‘Electrochemical properties of polyaniline-modified sodium vanadate nanomaterials’, Applied Physics A: Materials Science and Processing, 104(2), pp. 707–711. doi: 10.1007/s00339-011-6325-0.
Fuentes-Ríos, J. L. et al. (2021) ‘Modulation of the Pb/Sn ratio in Pb1-xSnxS thin films synthesized by chemical solution deposition’, Materials Science in Semiconductor Processing, 136(July). doi: 10.1016/j.mssp.2021.106126.
Geng, X. et al. (2022) ‘Tuning Phase Transition and Thermochromic Properties of Vanadium Dioxide Thin Films via Cobalt Doping’, ACS Applied Materials and Interfaces. doi: 10.1021/acsami.2c03113.
Hajzeri, M. et al. (2012) ‘Sol-gel vanadium oxide thin films for a flexible electronically conductive polymeric substrate’, Solar Energy Materials and Solar Cells, 99, pp. 62–72. doi: 10.1016/j.solmat.2011.03.041.
Ho, H. C. et al. (2019) ‘High quality thermochromic VO2 films prepared by magnetron sputtering using V2O5 target with in situ annealing’, Applied Surface Science, 495(July), p. 143436. doi: 10.1016/j.apsusc.2019.07.178.
Hryha, E., Rutqvist, E. and Nyborg, L. (2012) ‘Stoichiometric vanadium oxides studied by XPS’, Surface and Interface Analysis, 44(8), pp. 1022–1025. doi: 10.1002/sia.3844.
Hu, F. et al. (2017) ‘Synthesis and electrochemical performance of NaV6O15 microflowers for lithium and sodium ion batteries’, RSC Advances, 7(47), pp. 29481–29488. doi: 10.1039/c7ra04388k.
Ji, C. et al. (2018) ‘High thermochromic performance of Fe/Mg co-doped VO2 thin films for smart window applications’, Journal of Materials Chemistry C, 6(24), pp. 6502–6509. doi: 10.1039/c8tc01111g.
Khatibani, A. B., Abbasi, M. and Rozati, S. M. (2016) ‘Peculiarities of deposition times on gas sensing behaviour of vanadium oxide thin films’, Acta Physica Polonica A, 129(6), pp. 1245–1251. doi: 10.12693/APhysPolA.129.1245.
Li, B. et al. (2019) ‘Tungsten doped M-phase VO2 mesoporous nanocrystals with enhanced comprehensive thermochromic properties for smart windows’, Ceramics International, 45(4), pp. 4342–4350. doi: 10.1016/j.ceramint.2018.11.109.
Liu, S. et al. (2020) ‘One-step microwave-controlled synthesis of CoV2O6•2H2O nanosheet for super long cycle-life battery-type supercapacitor’, Electrochimica Acta, 364, p. 137320. doi: 10.1016/j.electacta.2020.137320.
Lu, C. et al. (2019) ‘Terahertz Transmittance of Cobalt-Doped VO2 Thin Film: Investigated by Terahertz Spectroscopy and Effective Medium Theory’, IEEE Transactions on Terahertz Science and Technology, 9(2), pp. 177–185. doi: 10.1109/TTHZ.2019.2894516.
Mane, A. A. and Moholkar, A. V. (2017) ‘Effect of film thickness on NO 2 gas sensing properties of sprayed orthorhombic nanocrystalline V 2 O 5 thin films’, Applied Surface Science, 416(2), pp. 511–520. doi: 10.1016/j.apsusc.2017.04.097.
Martínez-Gil, M. et al. (2020) ‘Effect of annealing temperature on the thermal transformation to cobalt oxide of thin films obtained via chemical solution deposition’, Materials Science in Semiconductor Processing, 107(October 2019). doi: 10.1016/j.mssp.2019.104825.
Peng, B. et al. (2018) ‘Transparent AlON ceramic combined with VO2 thin film for infrared and terahertz smart window’, Ceramics International, 44(12), pp. 13674–13680. doi: 10.1016/j.ceramint.2018.04.205.
Petnikota, S. et al. (2018) ‘Amorphous Vanadium Oxide Thin Films as Stable Performing Cathodes of Lithium and Sodium-Ion Batteries’, Nanoscale Research Letters, 13, pp. 1–13. doi: 10.1186/s11671-018-2766-0.
Sharma, G. P. et al. (2021) ‘Chalcogenide Dopant-Induced Lattice Expansion in Cobalt Vanadium Oxide Nanosheets for Enhanced Supercapacitor Performance’, ACS Applied Energy Materials, 4(5), pp. 4758–4771. doi: 10.1021/acsaem.1c00357.
Shen, N. et al. (2021) ‘Vanadium dioxide for thermochromic smart windows in ambient conditions’, Materials Today Energy, 21, p. 100827. doi: 10.1016/j.mtener.2021.100827.
Silversmit, G. et al. (2004) ‘Determination of the V2p XPS binding energies for different vanadium oxidation states (V5+ to V0+)’, Journal of Electron Spectroscopy and Related Phenomena, 135(2–3), pp. 167–175. doi: 10.1016/j.elspec.2004.03.004.
Silversmit, G. et al. (2006) ‘An XPS study on the surface reduction of V2O5(0 0 1) induced by Ar+ ion bombardment’, Surface Science, 600(17), pp. 3512–3517. doi: 10.1016/j.susc.2006.07.006.
Tabatabai Yazdi, S., Pilevar Shahri, R. and Shafei, S. (2021) ‘First synthesis of In-doped vanadium pentoxide thin films and their structural, optical and electrical characterization’, Materials Science and Engineering B: Solid-State Materials for Advanced Technology, 263(August 2020), p. 114755. doi: 10.1016/j.mseb.2020.114755.
Wang, S. et al. (2011) ‘Three-dimensional porous V 2O 5 cathode with ultra high rate capability’, Energy and Environmental Science, 4(8), pp. 2854–2857. doi: 10.1039/c1ee01172c.
Wang, S. et al. (2020) ‘Facile synthesis of VO2 (D) and its transformation to VO2(M) with enhanced thermochromic properties for smart windows’, Ceramics International, 46(10), pp. 14739–14746. doi: 10.1016/j.ceramint.2020.02.278.
Xu, Y. et al. (2019) ‘Ammonium Vanadium Bronze as a Potassium-Ion Battery Cathode with High Rate Capability and Cyclability’, Small Methods, 3(8), pp. 1–9. doi: 10.1002/smtd.201800349.
Yang, J. et al. (2010) ‘Synthesis and characterization of Cobalt hydroxide, cobalt oxyhydroxide, and cobalt oxide nanodiscs’, Journal of Physical Chemistry C, 114(1), pp. 111–119. doi: 10.1021/jp908548f.
Yao, X. et al. (2018) ‘Cesium-Doped Vanadium Oxide as the Hole Extraction Layer for Efficient Perovskite Solar Cells’, ACS Omega, 3(1), pp. 1117–1125. doi: 10.1021/acsomega.7b01944.
Yuan, L. et al. (2021) ‘In-Situ thermochromic mechanism of Spin-Coated VO2 film’, Applied Surface Science, 564(June), p. 150441. doi: 10.1016/j.apsusc.2021.150441.
Zhan, Y. et al. (2020) ‘Tuning thermochromic performance of VOx-based multilayer films by controlling annealing pressure’, Ceramics International, 46(2), pp. 2079–2085. doi: 10.1016/j.ceramint.2019.09.188.
Zhou, X. et al. (2020) ‘Abnormal dependence of microstructures and electrical properties of Y-doped VO2 thin films on deposition temperature’, Ceramics International, 46(11), pp. 18315–18321. doi: 10.1016/j.ceramint.2020.05.053.
Additional Files
Published
How to Cite
Issue
Section
License
Copyright (c) 2023

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
The journal Biotecnia is licensed under the Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0) license.




_(1)_(1).png)






_(2).jpg)





