Activity of starch biosynthetic enzymes and their association with endosperm modification in quality protein maize
DOI:
https://doi.org/10.18633/biotecnia.v27.2421Keywords:
quality protein maize , vitreous endosperm , starch biosynthesisAbstract
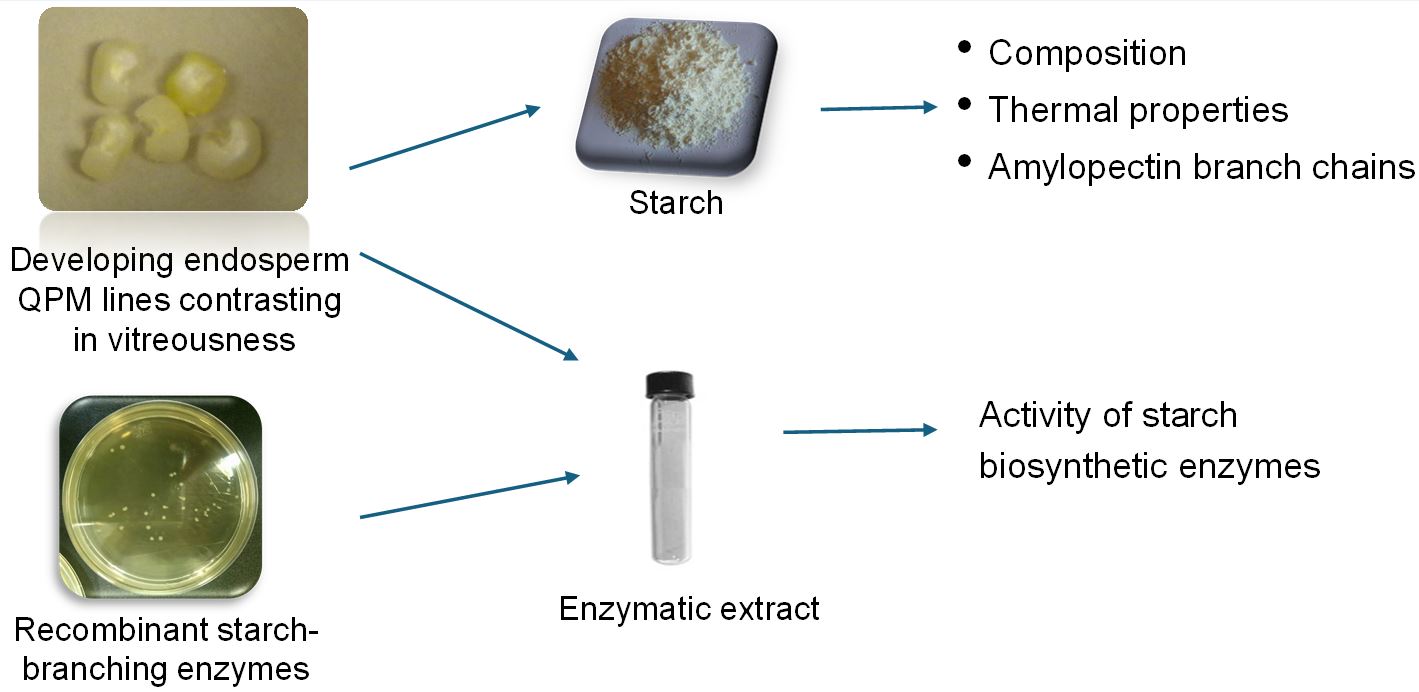
The formation of vitreous endosperm in quality protein maize (QPM) is associated with changes in the composition and structure of starch granules, but little is known about the role of alterations in the structure and activity of the main starch biosynthetic enzymes. Developing endosperms from K0326Y-QPM, W64Ao2, and derived recombinant inbred lines were used to analyze the activity of ADP-glucose pyrophosphorylase (AGPase), granule-bound starch synthase (GBSS), starch-branching enzyme (SBE) and pullulanase (PULL). GBSS activity correlated positively with kernel vitreousness and the opposite was observed for SBE. SBEIIb enzymes from K0326Y-QPM and W64Ao2 differed in five amino acids. Three amino acid changes appear to affect the SBEIIb catalytic site, which may be responsible for the lower activity and amylopectin content in vitreous lines. However, recombinant SBEIIb enzymes produced in Escherichia coli showed similar activities. The results suggest that endosperm modification in QPM is associated with changes in the activity of starch biosynthetic enzymes, which affect the starch composition, physicochemical and structural properties during endosperm development. The higher activity of GBSS and the lower activity of SBE produce starch granules with larger proportions of amylose and amorphous regions, favoring a greater compaction between these structures, contributing to the vitreous phenotype in QPM.
Downloads
References
Abad, M.C., Binderup, K., Rios-Steiner, J., Arni, R.K., Preiss, J. and Geiger, J.H. 2002. The X-ray crystallographic structure of Escherichia coli branching enzyme. Journal of Biological Chemistry 277: 42164-42170.
Baba, T., Kimura, K., Mizuno, K., Etoh, H., Ishida, Y., Shida, O. and Arai, Y. 1991. Sequence conservation of the catalytic regions of amylolytic enzymes in maize branching enzyme-I. Biochemical and Biophysical Research Communications 181: 87-94.
Chávez-Murillo, C.E., Méndez-Montealvo, G., Wang, Y.-J. and Bello-Pérez, L.A. 2012. Starch of diverse Mexican rice cultivars: physicochemical, structural, and nutritional features. Starch - Stärke 64: 745-756.
Chung, H.-J. and Liu, Q. 2009. Impact of molecular structure of amylopectin and amylose on amylose chain association during cooling. Carbohydrate Polymers 77: 807-815.
Dombrink-Kurtzman, M.A. and Knutson, C.A. 1997. A study of maize endosperm hardness in relation to amylose content and susceptibility to damage. Cereal Chemistry 74: 776-780.
FAOSTAT. 2024. Food and agriculture data. Food and Agriculture Organization of the United Nations. Rome, Italy: Food and Agriculture Organization of the United Nations. [Accessed 27 May 2024]. Available in http://faostat.fao.org.
Funane, K., Libessart, N., Stewart, D., Michishita, T. and Preiss, J. 1998. Analysis of essential histidine residues of maize branching enzymes by chemical modification and site-directed mutagenesis. Journal of Protein Chemistry 17: 579-590.
Gibbon, B.C., Wang, X. and Larkins, B.A. 2003. Altered starch structure is associated with endosperm modification in Quality Protein Maize. Proceedings of the National Academy of Sciences of the United States of America 100: 15329-15334.
Grimaud, F., Rogniaux, H., James, M.G., Myers, A.M. and Planchot, V. 2008. Proteome and phosphoproteome analysis of starch granule-associated proteins from normal maize and mutants affected in starch biosynthesis. Journal of Experimental Botany 59: 3395-3406.
Holding, D.R., Hunter, B.G., Klingler, J.P., Wu, S., Guo, X., Gibbon, B.C., Wu, R., Schulze, J.M., Jung, R. and Larkins, B.A. 2011. Characterization of opaque2 modifier QTLs and candidate genes in recombinant inbred lines derived from the K0326Y quality protein maize inbred. Theoretical and Applied Genetics 122: 783-794.
Kelley, L.A., Mezulis, S., Yates, C.M., Wass, M.N. and Sternberg, M.J. 2015. The Phyre2 web portal for protein modeling, prediction and analysis. Nature Protocols 10: 845-858.
Konik-Rose, C.M., Moss, R., Rahman, S., Appels, R., Stoddard, F. and Master, G.M. 2001. Evaluation of the 40 mg swelling test for measuring starch functionality. Starch/Stärke 53: 14–20.
Kuriki, T., Guan, H., Sivak, M. and Preiss, J. 1996. Analysis of the active center of branching enzyme II from maize endosperm. Journal of Protein Chemistry 15: 305-313.
Lee, H.J., Jee, M.G., Kim, J., Nogoy, F.M.C., Niño, M.C., Yu, D.A., Kim, M.S., Sun, M., Kang, K.K., Nou, I. and Cho, Y.G. 2014. Modification of starch composition using RNAi targeting soluble starch synthase I in Japonica rice. Plant Breeding and Biotechnology 2: 301-312.
Li, C., Xiang, X., Huang, Y., Zhou, Y., An, D., Dong, J., Zhao, C., Liu, H., Li, Y., Wang, Q., Du, C., Messing, J., Larkins, B.A., Wu, Y. and Wang, W. 2020. Long-read sequencing reveals genomic structural variations that underlie creation of quality protein maize. Nature Communications 11: 17.
Libessart, N. and Preiss, J. 1998. Arginine residue 384 at the catalytic center is important for branching enzyme II from maize endosperm. Archives of Biochemistry and Biophysics 360: 135-141.
Liu, F., Ahmed, Z., Lee, E.A., Donner, E., Liu, Q., Ahmed, R., Morell, M.K., Emes, M.J. and Tetlow, I.J. 2012. Allelic variants of the amylose extender mutation of maize demonstrate phenotypic variation in starch structure resulting from modified protein-protein interactions. Journal of Experimental Botany 63: 1167–1183.
Liu, F., Makhmoudova, A., Lee, E.A., Wait, R., Emes, M.J. and Tetlow, I.J. 2009. The amylose extender mutant of maize conditions novel protein–protein interactions between starch biosynthetic enzymes in amyloplasts. Journal of Experimental Botany 60: 4423-4440.
Liu, H., Shi, J., Sun, C., Gong, H., Fan, X., Qiu, F., Huang, X., Feng, Q., Zheng, X., Yuan, N., Li, C., Zhang, Z., Deng, Y., Wang, J., Pan, G., Han, B., Lai, J. and Wu, Y. 2016. Gene duplication confers enhanced expression of 27-kDa γ-zein for endosperm modification in quality protein maize. Proceedings of the National Academy of Sciences of the United States of America 113: 4964-4969.
Mertz, E.T., Bates, L.S. and Nelson, O.E. 1964. Mutant gene that changes protein composition and increases lysine content of maize endosperm. Science 145: 279-280.
Nishi, A., Nakamura, Y., Tanaka, N. and Satoh, H. 2001. Biochemical and genetic analysis of the effects of amylose-extender mutation in rice endosperm. Plant Physiology 127: 459-472.
Noguchi, J., Chaen, K., Vu, N.T., Akasaka, T., Shimada, H., Nakashima, T., Nishi, A., Satoh, H., Omori, T., Kakuta, Y. and Kimura, M. 2011. Crystal structure of the branching enzyme I (BEI) from Oryza sativa L with implications for catalysis and substrate binding. Glycobiology 21: 1108-1116.
Salazar-Salas, N.Y., Pineda-Hidalgo, K.V., Chavez-Ontiveros, J., Gutierrez-Dorado, R., Reyes-Moreno, C., Bello-Pérez, L.A., Larkins, B.A. and Lopez-Valenzuela, J.A. 2014. Biochemical characterization of QTLs associated with endosperm modification in quality protein maize. Journal of Cereal Science 60: 255-263.
Sambrook, J.F. and Russell, D. 2001. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
Tester, R.F. and Morrison, W.R. 1990. Swelling and gelatinization of cereal starches. I. Effects of amylopectin, amylose, and lipids. Cereal Chemistry 67: 551-557.
Tetlow, I.J. and Emes, M.J. 2014. A review of starch-branching enzymes and their role in amylopectin biosynthesis. IUBMB Life 66: 546-558.
Umemoto, T. and Terashima, K. 2002. Research note: Activity of granule-bound starch synthase is an important determinant of amylose content in rice endosperm. Functional Plant Biology 29: 1121-1124.
Vega-Alvarez, E., Pineda-Hidalgo, K.V., Salazar-Salas, N.Y., Soto-López, O.A., Canizalez-Roman, V.A., Garzón-Tiznado, J.A., Gutierrez-Dorado, R. and Lopez-Valenzuela, J.A. 2022. Genetic and molecular analysis of starch physicochemical properties and its relationship with endosperm modification in quality protein maize. Biotecnia XXIV: 140-149.
Villegas, E., Vasal, S.K., Bjarnason, M. and Mertz, E.T. 1992. Quality protein maize-what is it and how was it developed. In: Quality Protein Maize. E.T. Mertz (ed.), pp. 27-48. American Society of Cereal Chemists, Saint Paul, Minnesota.
Wu, H., Clay, K., Thompson, S.S., Hennen-Bierwagen, T.A., Andrews, B.J., Zechmann, B. and Gibbon, B.C. 2015. Pullulanase and starch synthase III are associated with formation of vitreous endosperm in quality protein maize. PloS One 10: e0130856.
Wu, Y., Holding, D.R. and Messing, J. 2010. γ-zeins are essential for endosperm modification in quality protein maize. Proceedings of the National Academy of Sciences of the United States of America 107: 12810-12815.
Yamanouchi, H. and Nakamura, Y. 1992. Organ specificity of isoforms of starch branching enzyme (Q-Enzyme) in rice. Plant and Cell Physiology 33: 985-991.
Yu, S., Ma, Y. and Sun, D.-W. 2009. Impact of amylose content on starch retrogradation and texture of cooked milled rice during storage. Journal of Cereal Science 50: 139-144.
Zhang, H.Y., Dong, S.T., Gao, R.Q. and Sun, Q.Q. 2007. Starch accumulation and enzyme activities associated with starch synthesis in maize kernels. Agricultural Sciences 6: 808-815.
Zhang, J.J., Hu, Y.F. and Huang, Y.B. 2008. Relationship between activities of key enzymes involved in starch synthesis and accumulation in maize inbred lines during grain filling. Russian Journal of Plant Physiology 55: 249-255.
Zhong, Y., Liu, L., Qu, J., Li, S., Blennow, A., Seytahmetovna, S.A., Liu, X. and Guo, D. 2020. The relationship between the expression pattern of starch biosynthesis enzymes and molecular structure of high amylose maize starch. Carbohydrate Polymers 247: 116681.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
The journal Biotecnia is licensed under the Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0) license.




_(1)_(1).png)






_(2).jpg)





