Comparative antimicrobial and antioxidant activity from collagen peptides from fish scales from Seriola rivoliana, using shrimp and commercial enzymes
DOI:
https://doi.org/10.18633/biotecnia.v27.2734Keywords:
collagen, peptides, antimicrobial, antioxidantAbstract
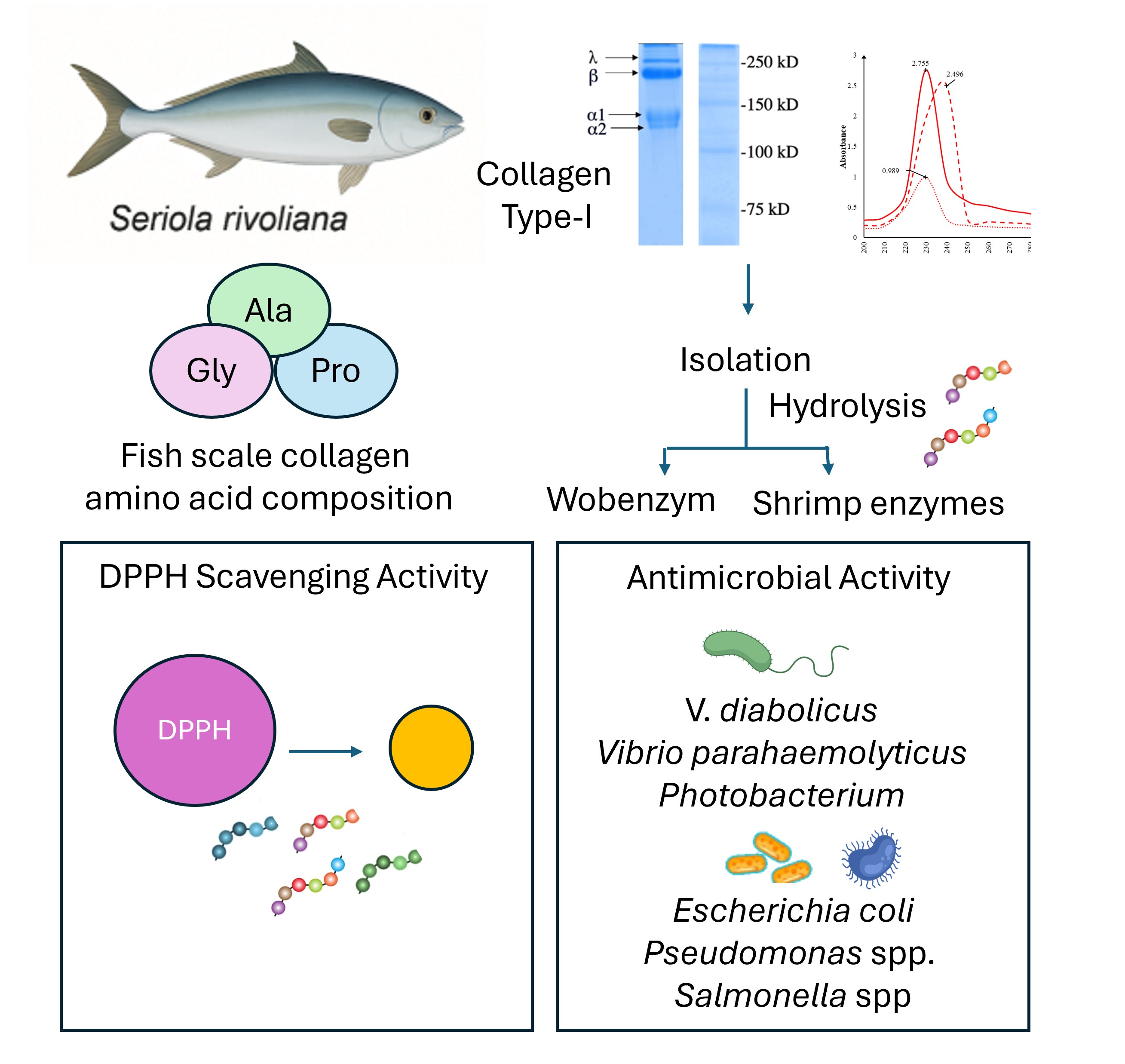
The fish processing industry faces dual challenges: environmental impact and low-profit applications for by-products, emphasizing the need to valorize this waste. In this study, collagen was isolated from Seriola rivoliana fish scales. SDS-PAGE results indicated that the purified collagen consisted of two distinct chains (α1- and α2-), consistent with the composition of type I collagen. Fish scale collagen is composed of Gly, Ala y Pro amino acids, with high proportion of Pro (20%), and exhibits an absorption peak at 230 nm in the UV-Vis spectrum. Collagen was hydrolyzed with 60 and 120 mU of activity of Wobenzym (WE) and digestive gland extract from shrimp waste (SE). The resulting collagen-derived peptides from WE showed DPPH scavenging activity, while shrimp-derived peptides did not. Both WE and SE-derived peptides inhibited the growth of marine pathogens (Vibrio diabolicus, Vibrio parahaemolyticus, and Photobacterium) and human pathogens (Escherichia coli, Pseudomonas spp., and Salmonella spp.). However, SE-derived peptides demonstrated stronger inhibitory effects against human pathogens, while WE-derived peptides were more effective against marine pathogens. These results suggest that waste materials, such as scales from the marine fish S. rivoliana, have potential as a source of collagen for generating peptides with antioxidant and antimicrobial properties.
Downloads
References
Ahmed, R., Haq, M. y Chun, B.S. 2019. Characterization of marine-derived collagen extracted from the by-products of bigeye tuna (Thunnus obesus). International Journal of Biological Macromolecules. 135: 668–676. https://doi.org/10.1016/j.ijbiomac.2019.05.213
Ali, A.M.M., Kishimura, H. y Benjakul, S. 2018. Extraction efficiency and characteristics of acid- and pepsin-soluble collagens from the skin of golden carp (Probarbus jullieni) as affected by ultrasonication. Process Biochemistry. 66: 237–244. https://doi.org/10.1016/j.procbio.2018.01.003
Atef, M., Chait, Y.A., Ojagh, S.M., Latifi, A.M., Esmaeili, M., Hammami, R. y Udenigwe, C.C. 2021. Anti-salmonella activity and peptidomic profiling of peptide fractions produced from sturgeon fish skin collagen (Huso huso) using commercial enzymes. Nutrients. 13(8): 2657. https://doi.org/10.3390/nu13082657
Bradford, M.M. 1976. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein dye binding. Analytical Biochemistry. 72: 248–254.
Chalamaiah, M., Kumar, B.D., Hemalatha, R. y Jyothirmayi, T. 2012. Fish protein hydrolysates: Proximate composition, amino acid composition, antioxidant activities and applications: A review. Food Chemistry. 135(4): 3020–3038. https://doi.org/10.1016/j.foodchem.2012.06.100
Chen, J., Li, L., Yi, R., Xu, N., Gao, R. y Hong, B. 2016. Extraction and characterization of acid-soluble collagen from scales and skin of tilapia (Oreochromis niloticus). LWT - Food Science and Technology. 66: 453–459. https://doi.org/10.1016/j.lwt.2015.10.070
Chen, S., Chen, H., Xie, Q., Hong, B., Chen, J., Hua, F., Bai, K., He, J., Yi, R. y Wu, H. 2016. Rapid isolation of high-purity pepsin-soluble type I collagen from scales of red drum fish (Sciaenops ocellatus). Food Hydrocolloids. 52: 468–477. https://doi.org/10.1016/j.foodhyd.2015.07.027
Chinh, N.T., Manh, V.Q., Trung, V.Q., et al. 2019. Characterization of collagen derived from tropical freshwater carp fish scale wastes and its amino acid sequence. Natural Product Communications. 14(7). https://doi.org/10.1177/1934578X19866288
Ennaas, N., Hammami, R., Gomaa, A., Bédard, F., Biron, É., Subirade, M., Beaulieu, L. y Fliss, I. 2016. Collagencin, an antibacterial peptide from fish collagen: Activity, structure and interaction dynamics with membrane. Biochemical and Biophysical Research Communications. 473(2): 642–647. https://doi.org/10.1016/j.bbrc.2016.03.121
Ennaas, N., Hammami, R., Beaulieu, L. y Fliss, I. 2015. Production of antibacterial fraction from Atlantic mackerel (Scomber scombrus) and its processing byproducts using commercial enzymes. Food and Bioproducts Processing. 96: 145–153. https://doi.org/10.1016/j.fbp.2015.07.014
García-Carreño, F.L. y Haard, N.F. 1993. Characterization of proteinase classes in langostilla (Pleuroncodes planipes) and crayfish (Pacifastacus astacus) extracts. Journal of Food Biochemistry. 17: 97–113. https://doi.org/10.1111/j.1745-4514.1993.tb00864.x
Gratzfeld-Huesgen, A. 1999. Sensitive and reliable amino acids analysis in protein hydrolysates using the Agilent 1100 Series HPLC. Agilent Technologies Technical Note.
Huang, C.Y., Kuo, J.M., Wu, S.J. y Tsai, H.T. 2016. Isolation and characterization of fish scale collagen from tilapia (Oreochromis sp.) by a novel extrusion–hydro-extraction process. Food Chemistry. 190: 997–1006. https://doi.org/10.1016/j.foodchem.2015.06.066
Jerez, H.S. 2013. Cultivo de carángidos: La Seriola y el Jurel Dentón. En: Diversificación de especies en la piscicultura marina española. Publicaciones científicas y tecnológicas de la Fundación Observatorio Español de Acuicultura.
Laemmli, U.K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 227(5259): 680–685. https://doi.org/10.1038/227680a0
Lukin, I., Erezuma, I., Maeso, L., Zarate, J., Desimone, M.F., Al-Tel, T.H., Dolatshahi-Pirouz, A. y Orive, G. 2022. Progress in gelatin as biomaterial for tissue engineering. Pharmaceutics. 14: 1177. https://doi.org/10.3390/pharmaceutics14061177
Minh Thuy, L.T., Okazaki, E. y Osako, K. 2014. Isolation and characterization of acid-soluble collagen from the scales of marine fishes from Japan and Vietnam. Food Chemistry. 149: 264–270. https://doi.org/10.1016/j.foodchem.2013.10.094
Montero, P. y Gomez-Guillen, M.C. 2000. Extracting conditions for megrim (Lepidorhombus boscii) skin collagen affect functional properties of the resulting gelatin. Journal of Food Science. 65: 434–438. https://doi.org/10.1111/j.1365-2621.2000.tb16022.x
Pati, F., Datta, P., Adhikari, B., Dhara, S., Ghosh, K. y Mohapatra, P.K.D. 2012. Collagen scaffolds derived from freshwater fish origin and their biocompatibility. Journal of Biomedical Materials Research Part A. 100(4): 1068–1079. https://doi.org/10.1002/jbm.a.33280
Rojo-Arreola, L., del Toro, M.A.N., Cordova-Murueta, J., et al. 2019. Techniques for protein digestion research in Decapoda: A review. Trends in Food Science and Technology. https://doi.org/10.1016/j.tifs.2019.05.004
Shimizu, J., Shimizu, H., Nagashima, K., Yamada, K. y Takamashu, M. 2001. Fish collagen and method of producing same. U.S. Patent 6,271,350.
Shoulders, M.D. y Raines, R.T. 2009. Collagen structure and stability. Annual Review of Biochemistry. 78: 929–958. https://doi.org/10.1146/annurev.biochem.77.032207.120833
Ulzanah, N., Wahjuningrum, D., Widanarni, W. y Kusumaningtyas, E. 2023. Peptide hydrolysate from fish skin collagen to prevent and treat Aeromonas hydrophila infection in Oreochromis niloticus. Veterinary Research Communications. 47(2): 487–494. https://doi.org/10.1007/s11259-022-09969-6
Upasen, S., Naeramitmarnsuk, K., Antonio, C., Roces, S., Morillas, H. y Wattanachai, P. 2019. Acid-pepsin soluble collagen from saltwater and freshwater fish scales. Engineering Journal. 23(5): 183–195.
Veyrand-Quirós, B., Gómez-Gil, B., Lomelí-Ortega, C.O., Escobedo-Fregoso, C., Millard, A.D., Tovar-Ramírez, D., Balcázar, J.L. y Quiroz-Guzmán, E. 2020. Use of bacteriophage vB_Pd_PDCC-1 as biological control agent of Photobacterium damselae subsp. damselae during hatching of longfin yellowtail (Seriola rivoliana) eggs. Journal of Applied Microbiology. 129(6): 1497–1510. https://doi.org/10.1111/jam.1474
Wang, B., Wang, Y.M., Chi, C.F., et al. 2013. Isolation and characterization of collagen and antioxidant collagen peptides from scales of croceine croaker (Pseudosciaena crocea). Marine Drugs. 11: 4641–4661. https://doi.org/10.3390/md11114641
Wahyu, Y.I. y Widjanarko, S.B. 2018. Extraction optimization and characterization of acid-soluble collagen from milkfish scales (Chanos chanos Forksal). Carpathian Journal of Food Science and Technology. 10(1): 125–135.
Yan, M., Li, B., Zhao, X., Ren, G., Zhuang, Y., Hou, H., Zhang, X., Chen, L. y Fan, Y. 2008. Characterization of acid-soluble collagen from the skin of walleye pollock (Theragra chalcogramma). Food Chemistry. 107(4): 1581–1586. https://doi.org/10.1016/j.foodchem.2007.10.027
Zhang, F., Wang, A., Li, Z., He, S. y Shao, L. 2011. Preparation and characterization of collagen from freshwater fish scales. Food and Nutrition Sciences. 2: 818–823. https://doi.org/10.4236/fns.2011.28112
Zamorano-Apodaca, J.C., García-Sifuentes, C.O., Carvajal-Millán, E., Vallejo-Galland, B., Scheuren-Acevedo, S.M. y Lugo-Sánchez, M.E. 2020. Biological and functional properties of peptide fractions obtained from collagen hydrolysate derived from mixed by-products of different fish species. Food Chemistry. 331: 127350. https://doi.org/10.1016/j.foodchem.2020.127350
Published
How to Cite
Issue
Section
License
Copyright (c) 2025

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
The journal Biotecnia is licensed under the Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0) license.
















_(2).jpg)







