Comprehensive characterization of the overlooked residue generated during roselle calyxes brewing with potential use as functional ingredient
DOI:
https://doi.org/10.18633/biotecnia.v25i3.2153Keywords:
Hibiscus sabdariffa L., subproducto, espectrometría de masasAbstract
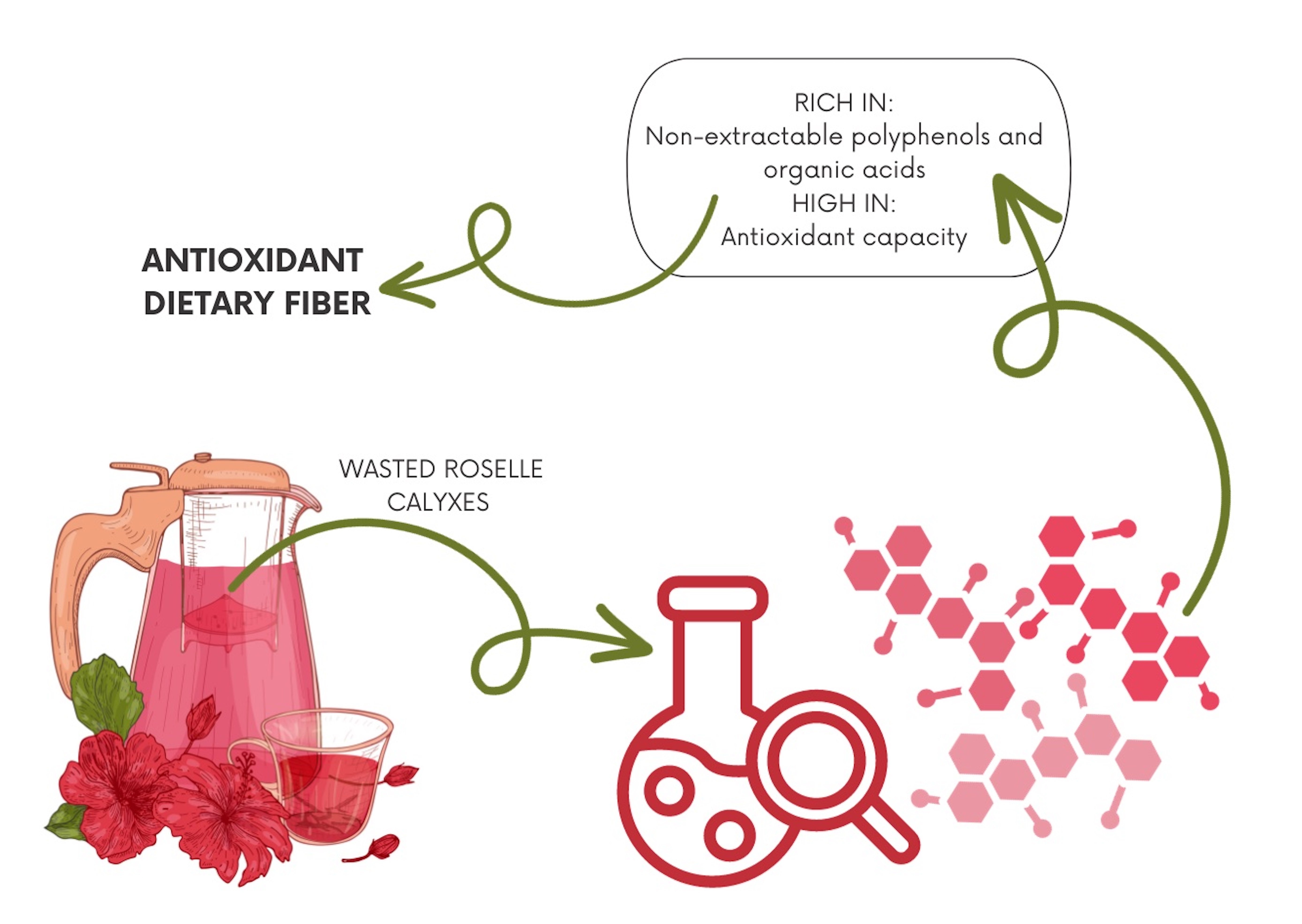
A significant quantity Hibiscus sabdariffa L. calyxes is generated as a by-product during the decoction process commonly used for roselle beverage preparation. We conducted an extensive characterization of polyphenols, organic acids, and antioxidant potential in roselle calyxes, decoction, and their by-product. Roselle calyxes were found to be a rich source of diverse polyphenols, including delphinidin sambubioside, caffeoylquinic acids, hibiscus acid, and citric acid as major components. Importantly, we extracted a significant proportion of these bioactive compounds during the decoction process, resulting in a polyphenol-rich beverage. The used calyces (decoction by-product) retained from 23 % to 140 % of the extractable polyphenols and organic acids found in roselle calyxes. Additionally, due to the leaching of hydrophobic components like soluble dietary fiber and extractable polyphenols and organic acids, the roselle by-product was enriched with non-extractable constituents attached to dietary fiber (126 % - 272 %). Therefore, roselle calyxes and their decoction by-products emerge as promising sources of polyphenols with the potential for use in dietary supplements, alongside the commonly consumed roselle decoction.
Downloads
References
Amaya-Cruz, D., Peréz-Ramírez, I.F., Pérez-Jiménez, J., Nava, G.M. and Reynoso-Camacho, R. 2019. Comparison of the bioactive potential of Roselle (Hibiscus sabdariffa L.) calyx and its by-product: Phenolic characterization by UPLC-QTOF MSE and their anti-obesity effect in vivo. Food Research International. 126: 108589.
Amaya-Cruz, D.M., Perez-Ramirez, I.F., Ortega-Diaz, D., Rodriguez-Garcia, M.E. and Reynoso-Camacho, R. (2018). Roselle (Hibiscus sabdariffa) by-product as functional ingredient: effect of thermal processing and particle size reduction on bioactive constituents and functional, morphological, and structural properties. Journal of Food Measurement and Characterization. 12: 135-144.
Báez‐García, E., Sáyago‐Ayerdi, S.G. and Pérez‐Jiménez, J. 2023. Non‐extractable polyphenols should be systematically included in polyphenol analysis. Recent Advances in Polyphenol Research. 8: 193-238.
Bedi, R., Bedi, S. and Chawla, P. 2020. Effect of pH on the extraction of anthocyanin from roselle (Hibiscus sabdariffa L.) calyces. Journal of Food Processing and Preservation. 44: e14962.
Bhadra, P. (2020). In-silico analysis of Roselle (Hibiscus sabdariffa L.) for Antidiabetic. Indian Journal of Natural Sciences. 10: 20764-20768.
Clifford, M.N., Jaganath, I.B., Ludwig, I.A., Crozier, A. 2017. Chlorogenic acids and the acyl-quinic acids: discovery, biosynthesis, bioavailability and bioactivity. Natural Product Reports. 34: 1391-1421.
Da-Costa-Rocha, I., Bonnlaender, B., Sievers, H., Pischel, I. and Heinrich, M. 2014. Hibiscus sabdariffa L.–a phytochemical and pharmacological review. Food Chemistry. 165: 424-443.
Debnath, B., Haldar, D. and Purkait, M.K. 2021. Potential and sustainable utilization of tea waste: A review on present status and future trends. Journal of Environmental Chemical Engineering, 9: 106179.
Ding, Y., Morozova, K., Scampicchio, M. and Ferrentino, G. 2020. Non-Extractable polyphenols from food by-products: Current knowledge on recovery, characterisation, and potential applications. Pro-cesses. 8: 925.
Dong, L., Li, S., Zhang, D., Li, X., Wang, X. and He, Z. 2021. Structural characterization of dietary fiber–polyphenol complexes and their influence on polyphenol bioaccessibility in carrots using high-resolution mass spectrometry. Food Chemistry. 335: 127596.
Firuzi, O., Lacanna, A., Petrucci, R., Marrosu, G. and Saso, L. 2005. Evaluation of the antioxidant activity of flavonoids by “ferric reducing antioxidant power” assay and cyclic voltammetry. Biochimica et Biophysica Acta (BBA)-General Subjects. 1721: 174-184.
Fukumoto, L.R., Mazza, G. 2000. Assessing antioxidant and prooxidant activities of phenolic compounds. Journal of Agricultural and Food Chemistry. 48: 3597-3604.
Giusti, M.M. and Wrolstad, R.E. 2001. Characterization and measurement of anthocyanins by UV‐visible spectroscopy. Current Protocols in Food Analytical Chemistry. 1: F1-2.
Hassan F.A., Ismail A., Hamid A.A., Azlan A., Al-Sheraji S.H. 2011. Characterization of fibre-rich powder and antioxidant capacity of Mangifera pajang K. fruit peels. Food Chemistry. 126: 283–288.
Hinojosa-Gómez, J., San Martín-Hernández, C., Heredia, J. B., León-Félix, J., Osuna-Enciso, T. and Muy-Rangel, M. D. (2020). Anthocyanin induction by drought stress in the calyx of roselle cultivars. Molecules, 25: 1555.
Izquierdo-Vega, J. A., Arteaga-Badillo, D. A., Sánchez-Gutiérrez, M., Morales-González, J. A., Vargas-Mendoza, N., Gómez-Aldapa, C. A., Castro-Rosas, J., Delgado-Olivares, L., Madrigal-Bujaidar, E. and Madrigal-Santillán, E. 2020. Organic acids from Roselle (Hibiscus sabdariffa L.)—A brief review of its pharmacological effects. Biomedicines. 8: 100.
Jaiswal, Y., Liang, Z. and Zhao, Z. 2016. Botanical drugs in Ayurveda and traditional Chinese medicine. Journal of Ethnopharmacology. 194: 245-259.
Jamrozik, D., Borymska, W. and Kaczmarczyk-Żebrowska, I. 2022. Hibiscus sabdariffa in Diabetes Prevention and Treatment—Does It Work? An Evidence-Based Review. Foods. 11: 2134.
Kakkar, S. and Bais, S. 2014. A review on protocatechuic acid and its pharmacological potential. Interna-tional Scholarly Research Notices. 2014: 952943.
Lin, H., Tello, E., Simons, C.T. and Peterson, D.G. 2022. Identification of subthreshold chlorogenic acid lactones that contribute to flavor instability of ready-to-drink coffee. Food Chemistry. 395: 133555.
Long, Q., Chen, H., Yang, W., Yang, L. and Zhang, L. 2021. Delphinidin-3-sambubioside from Hibiscus sabdariffa. L attenuates hyperlipidemia in high fat diet-induced obese rats and oleic acid-induced ste-atosis in HepG2 cells. Bioengineered. 12: 3837-3849.
Mercado-Mercado, G., Blancas-Benitez, F.J., Velderrain-Rodríguez, G.R., Montalvo-González, E., González-Aguilar, G.A., Alvarez-Parrilla, E. and Sáyago-Ayerdi, S.G. 2015. Bioaccessibility of polyphenols released and associated to dietary fibre in calyces and decoction residues of Roselle (Hibiscus sabdariffa L.). Journal of Functional Foods. 18: 171-181.
Morales‐Luna, E., Pérez‐Ramírez, I. F., Salgado, L. M., Castaño‐Tostado, E., Gómez‐Aldapa, C. A. and Reynoso‐Camacho, R. (2019). The main beneficial effect of roselle (Hibiscus sabdariffa) on obesity is not only related to its anthocyanin content. Journal of the Science of Food and Agriculture. 99: 596-605.
Oomah, B.D., Cardador‐Martínez, A. and Loarca‐Piña, G., 2005. Phenolics and antioxidative activities in common beans (Phaseolus vulgaris L). Journal of the Science of Food and Agriculture. 85: 935-942.
Quatrin, A., Pauletto, R., Maurer, L.H., Minuzzi, N., Nichelle, S.M., Carvalho, J.F.C., Maróstica, M.R.., Rodriguez, E., Bochi, V.C. and Emanuelli, T. 2019. Characterization and quantification of tannins, flavonols, anthocyanins and matrix-bound polyphenols from jaboticaba fruit peel: A comparison between Myrciaria trunciflora and M. jaboticaba. Journal of Food Composition and Analysis. 78: 59-74.
Rababah, T. M., Ereifej, K. I., Esoh, R. B., Al-u'datt, M. H., Alrababah, M. A. and Yang, W. 2011. An-tioxidant activities, total phenolics and HPLC analyses of the phenolic compounds of extracts from common Mediterranean plants. Natural Product Research. 25: 596-605.
Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M. and Rice-Evans, C. 1999. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology and Medicine. 26:1231-1237.
Reynoso-Camacho, R., Sotelo-González, A.M., Patiño-Ortiz, P., Rocha-Guzmán, N.E. and Pérez-Ramírez, I.F. (2021). Berry by-products obtained from a decoction process are a rich source of low-and high-molecular weight extractable and non-extractable polyphenols. Food and Bioproducts Processing. 127: 371-387.
Ríaz, E. and Chopra, D. (2018). Roselle (Hibiscus sabdariffa L.) seed protein isolate: extraction optimi-zation and physicochemical properties. Food Chemistry. 240: 1141-1148.
Rothwell, J.A., Pérez-Jiménez, J., Neveu, V., Medina-Ramon, A., M’Hiri, N., Garcia-Lobato, P., Manach, C., Knox, K., Eisner, R., Wishart, D. and Scalbert, A. (2013). Phenol-Explorer 3.0: a major update of the Phenol-Explorer database to incorporate data on the effects of food processing on a polyphenol content. Database. 2013: bat070.
Salem, M. A., Michel, H. E., Ezzat, M. I., Okba, M. M., El-Desoky, A. M., Mohamed, S. O. and Ezzat, S. M. 2020. Optimization of an extraction solvent for angiotensin-converting enzyme inhibitors from Hibiscus sabdariffa L. based on its UPLC-MS/MS metabolic profiling. Molecules. 25: 2307.
Sapian, S., Ibrahim Mze, A.A., Jubaidi, F.F., Mohd Nor, N.A., Taib, I. S., Abd Hamid, Z., Zainalabidin, S., Anuar, N.N.M., Katas, H., Latip, J., Jalil, J., Abu Bakar, N.F. and Budin, S.B. 2023. Therapeutic potential of Hibiscus sabdariffa Linn. in attenuating cardiovascular risk factors. Pharmaceuticals. 16: 807.
Sáyago‐Ayerdi, S.G., Velázquez‐López, C., Montalvo‐González, E. and Goñi, I. 2014. By‐product from decoction process of Hibiscus sabdariffa L. calyces as a source of polyphenols and dietary fiber. Journal of the Science of Food and Agriculture. 94: 898-904.
Selli, S., Cabaroglu, T., and Canbas, A. 2021. The aroma of Turkish red rose (Rosa damascena Mill.) hydrosols. Food Chemistry. 346: 128954.
Singleton, V.L., Orthofer, R. and Lamuela-Raventós, R.M., 1999. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods in Enzymology. 299: 152-178.
Trinh, L. T. P., Choi, Y. S., & Bae, H. J. (2018). Production of phenolic compounds and biosugars from flower resources via several extraction processes. Industrial Crops and Products. 125: 261-268.
Yang, D., Xu, H. X., Guo, Y. X. and Zhang, Q. F. 2023. Chemical profile of Roselle extract and its in-hibitory activities on three digestive enzymes in vitro and in vivo. International Journal of Biological Macromolecules. 253: 126902.
Zurita, J., Díaz-Rubio, M.E., and Saura-Calixto, F. 2012. Improved procedure to determine non-extractable polymeric proanthocyanidins in plant foods. International Journal of Food Sciences and Nutrition. 63: 936–939.
Additional Files
Published
How to Cite
Issue
Section
License
Copyright (c) 2023

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
The journal Biotecnia is licensed under the Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0) license.






_(1)_(1).png)






_(2).jpg)




