Characterization, antioxidant capacity and erythroprotective effect of Zaya (Amoreuxia palmatifida) extracts
DOI:
https://doi.org/10.18633/biotecnia.v27.2547Keywords:
antioxidants, secondary metabolites, erythrocytesAbstract
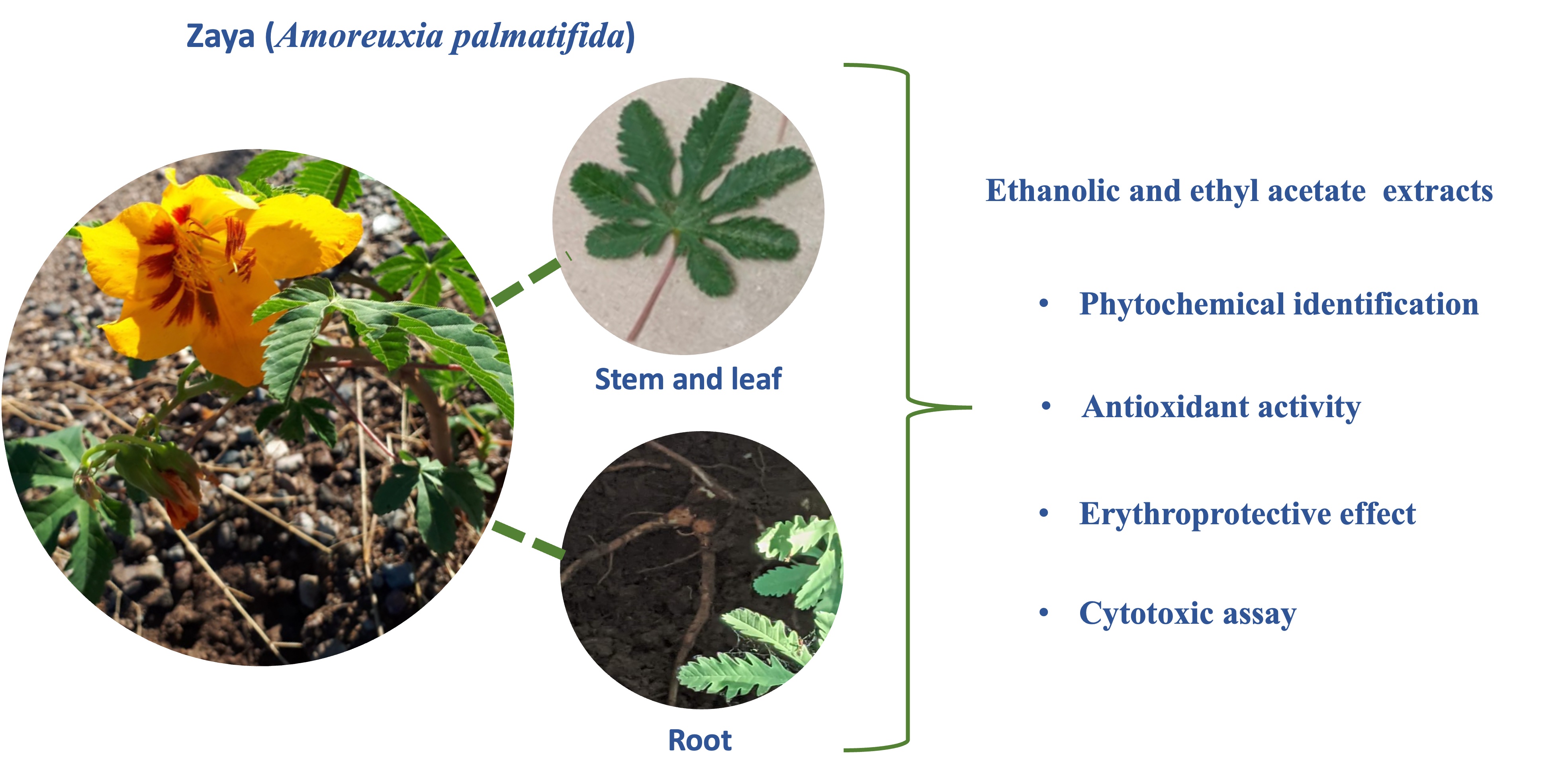
Zaya (Amoreuxia palmatifida) is a herbaceous plant found in northwest Mexico, particularly in the state of Sonora, where it has been historically consumed by its first settlers due to its nutritional and medicinal properties. Despite its importance, there is little information about the phytochemical compounds present in Zaya, its antioxidant activity and erythroprotective effect. Therefore, this study aimed to identify and quantify some of the compounds of interest related to the antioxidant capacity and erythroprotective effect of the plant. The results show that ethanolic extracts of zaya leaf (LE), root (RE) and stem (SE) contain the highest amount of phenols, tannins, flavonoids, chlorophylls and carotenoids, compared to extracts with leaf ethyl acetate (LEA), root (REA), and stem (SEA). Antioxidant activity was highest in LE by DPPH (61.27 ± 1.03 % of inhibition), ABTS (58.21 ± 0.48 % of inhibition) and FRAP (132.44 ± 5.23). All Zaya extracts had erythroprotective effect on O+ (80 to 87 %), followed by B+ (22 - 85 %) and then with A+ (38 - 60 %). In the cytotoxic assay, the highest percentage of hemolysis (41 - 50 %) occurred in blood type B+ with REA, RE, LEA and LE, while the lowest was in blood type O+. Therefore, LE contains the best secondary metabolites that confer greater antioxidant and erythroprocteric capacity without causing toxicity.
Downloads
References
Agarwal, H., & Shanmugam, V. K. (2019). Anti-inflammatory activity screening of Kalanchoe pinnata methanol extract and its validation using a computational simulation approach. Informatics in Medicine Unlocked, 14, 6-14.
Ahmad, M. H., Jatau, A. I., Khalid, G. M., & Alshargi, O. Y. (2021). Traditional uses, phytochemistry, and pharmacological activities of Cochlospermum tinctorium A. Rich (Cochlospermaceae): a review. Future Journal of Pharmaceutical Sciences, 7, 1-13.
Alcaráz-López, O. A. (2009). Determinación de la actividad antioxidante y antiinflamatoria in Vitro de las plantas medicinales Heterotheca inuloides, Calea urticifolia, Buddleia spp. y Sambucus spp. usadas en el estado de Jalisco. Tesis de Maestría presentada en el Centro Universitario de la Ciénega, Universidad de Guadalajara. Pp. 48-49
Barlow, S. M. (1990). Toxicological aspects of antioxidants used as food additives. Food antioxidants, 253-307.
Benzie, I., Strain, J., 1996. The ferric reducing ability of plasma (FRAP) as a measure of ‘‘antioxidant power”: The FRAP assay. Anal. Biochem. 239, 70–76. https://doi. org/10.1006/abio.1996.0292.
Brand-Williams, W., Cuvelier, M., Berset, C., 1995. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 28, 25–30. https://doi.org/ 10.1016/S0023-6438(95)80008-5.
Cameron, S. I., Smith, R. F., & Kierstead, K. E. (2005). Linking medicinal/nutraceutical products research with commercialization. Pharmaceutical biology, 43(5), 425-433.
Castro Montoya, J. A., Zayas Barreras, R. A., Saiz Aguilar, P., Romero Lozoya, M., Bojorquez Camacho, F. R., & Bojorquez Camacho, O. (2012). El consumo de la zaya (Amoreuxia spp) una tradición cultural de la región del Évora en el estado de Sinaloa, México. Revista Mexicana de Agronegocios, 30(1345-2016-104232), 898-907.
Celaya-Michel, H., Ochoa-Meza, A., López-Elías, J., & Barrera-Silva, M. Á. (2017). Germinación y crecimiento en vivero y en campo de zaya (Amoreuxia palmatifida DC.), una especie nativa amenazada en México. European Scientific Journal, 13, 66-78.
Cooling, L. (2015). Blood groups in infection and host susceptibility. Clinical microbiology reviews, 28(3), 801-870.
García, CM. Kim, P. Bich, NB. Tillan, NT. Romero, J. C. Dario, JA. y Fuste, V.M. 2009. Metabolitos secundarios en los extractos secos de Passiflora Incarnata L, Matricaria recutita L. y Morinda citrifolia L. Revista Cubana de Plantas Medicinales. 14(2): 1-5.
Galindo, W. F., Rosales, M., Murgueitio, E., & Larrahondo, J. (1989). Sustancias antinutricionales en las hojas de guamo, nacedero y matarratón. Livestock research for rural development, 1(1), 36-47.
González-Gallego, J., García-Mediavilla, M. V., Sánchez-Campos, S., & Tuñón, M. J. (2010). Fruit polyphenols, immunity and inflammation. British journal of nutrition, 104(S3), S15-S27.
Guajardo-Flores, D., García-Patiño, M., Serna-Guerrero, D., Gutiérrez-Uribe, J. A., & Serna-Saldívar, S. O. (2012). Characterization and quantification of saponins and flavonoids in sprouts, seed coats and cotyledons of germinated black beans. Food chemistry, 134(3), 1312-1319.
Hörtensteiner, S. (2006). Chlorophyll degradation during senescence. Annu. Rev. Plant Biol., 57, 55-77.
Hodgson, W. (1993). Bixaceae, lipstick tree family. Journal of the Arizona -Nevada Academy of Science, 27, 188-189.
Isaksson, C. (2009). The chemical pathway of carotenoids: from plants to birds. Ardea, 97(1), 125-128.
Jeffrey, S.W., Humphrey, G.F., 1975. New Spectrophotometric Equations for Determining Chlorophylls a, b c1 and c2 in Higher Plants, Algae and Natural Phytoplankton. Biochem. Physiol. Pflanz. (BPP) 167, 191–194. https://doi.org/ 10.1016/S0015-3796(17)30778-3.
Lee, J., Kwak, M., Chang, Y. K., & Kim, D. (2021). Green solvent-based extraction of chlorophyll a from Nannochloropsis sp. Using 2, 3-butanediol. Separation and Purification Technology, 276, 119248.
Lu, J. et al., 2010. Flavonoids from the leaves of Actinidia kolomikta. Chem. Nat. Compd. 46, 205–208 https://link.springer.com/article/10.1007/s10600-010- 9569-6.
Lobo, V., Patil, A., Phatak, A., & Chandra, N. (2010). Free radicals, antioxidants and functional foods: Impact on human health. Pharmacognosy reviews, 4(8), 118.
Michel, H. C., Islas, J. D. R. R., Hinojo, C. H., Rosas, M. C., & Silva, M. B. (2023). Perspectivas del cultivo de la saya (Amoreuxia spp.) en el noroeste de México como nuevo producto agronómico. Agricultura, Sociedad y Desarrollo, 20(2), 4.
Müller, L., Fröhlich, K., & Böhm, V. (2011). Comparative antioxidant activities of carotenoids measured by ferric reducing antioxidant power (FRAP), ABTS bleaching assay (αTEAC), DPPH assay and peroxyl radical scavenging assay. Food Chemistry, 129(1), 139-148.
Oumar, Y. S., Nathalie, G. K., Karamoko, O., Alexis, B. G., & Adama, C. (2014). In vitro antioxidant activity of extracts of the root Cochlospermum planchonii Hook. f. ex. Planch (Cochlospermaceae). Journal of Pharmacognosy and Phytochemistry, 3(4), 164-170.
Palta, J. P. (1990). Leaf chlorophyll content. Remote sensing reviews, 5(1), 207-213.
Pandey, K. B., & Rizvi, S. I. (2009). Plant polyphenols as dietary antioxidants in human health and disease. Oxidative medicine and cellular longevity, 2, 270-278.
Pant, P., Pandey, S., & Dall'Acqua, S. (2021). The influence of environmental conditions on secondary metabolites in medicinal plants: A literature review. Chemistry & Biodiversity, 18(11), e2100345.
Poppendieck, H. H. (1981). Cochlospermaceae. Flora Neotropica, 27, 1-33.
Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M., Rice-Evans, C., 1999. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Rad. Biol. Med. 10, 1231–1237. https://doi.org/10.1016/S0891-5849 (98)00315-3
Roshanak, S., Rahimmalek, M., & Goli, S. A. H. (2016). Evaluation of seven different drying treatments in respect to total flavonoid, phenolic, vitamin C content, chlorophyll, antioxidant activity and color of green tea (Camellia sinensis or C. assamica) leaves. Journal of food science and technology, 53, 721-729.
Sánchez, Y.G., Rondón, L.A., Hermosilla, R.E. y Almeida, M.S. 2010. Tamizaje fitoquímico de los extractos alcohólico, etéreo y acuoso de las hojas, tallos y flores de Helychrysum bracteatum. Revista Química Viva. 1: 40-45.
Secretaría de Medio Ambiente y Recursos Naturales (SEMARNAT). (2010). Norma Oficial Mexicana NOM-059-SEMARNAT-2010, Protección ambiental - Especies nativas de México de flora y fauna silves-tres - Categorías de riesgo y especificaciones para su inclusión, exclusión o cambio - Lista de especies en riesgo. Ciudad de México: SEMARNAT.
Sui, X. L., Mao, S. L., Wang, L. H., Zhang, B. X., & Zhang, Z. X. (2012). Effect of low light on the characteris-tics of photosynthesis and chlorophyll a fluorescence during leaf development of sweet pepper. Journal of Integrative Agriculture, 11(10), 1633-1643.
Tiwari, B.K., Brunton, N. and Brennan, C.S. 2015. Handbook of plant food phytochemicals. Wiley-Blackwell. Oxford, UK
Thirunavukarasu, A.J., Ross, A.C. and Gilbert, R.M. 2022. Vitamin A, systemic T-cells, and the eye: Focus on degenerative retinal disease. Frontiers in Nutrition. 9: 914457.
WRB. 2022. World Reference Base for Soil Resources 2014, Update 2022. World Soil Resources Reports No. 106. FAO, Roma, Italia.
Zhang, Y., Cai, P., Cheng, G. and Zhang, Y. 2022. A brief review of phenolic compounds identified from plants: Their extraction, analysis, and biological activity. Natural Product Communications. 17(1): 1934578X211069721.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
The journal Biotecnia is licensed under the Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0) license.
















_(2).jpg)







